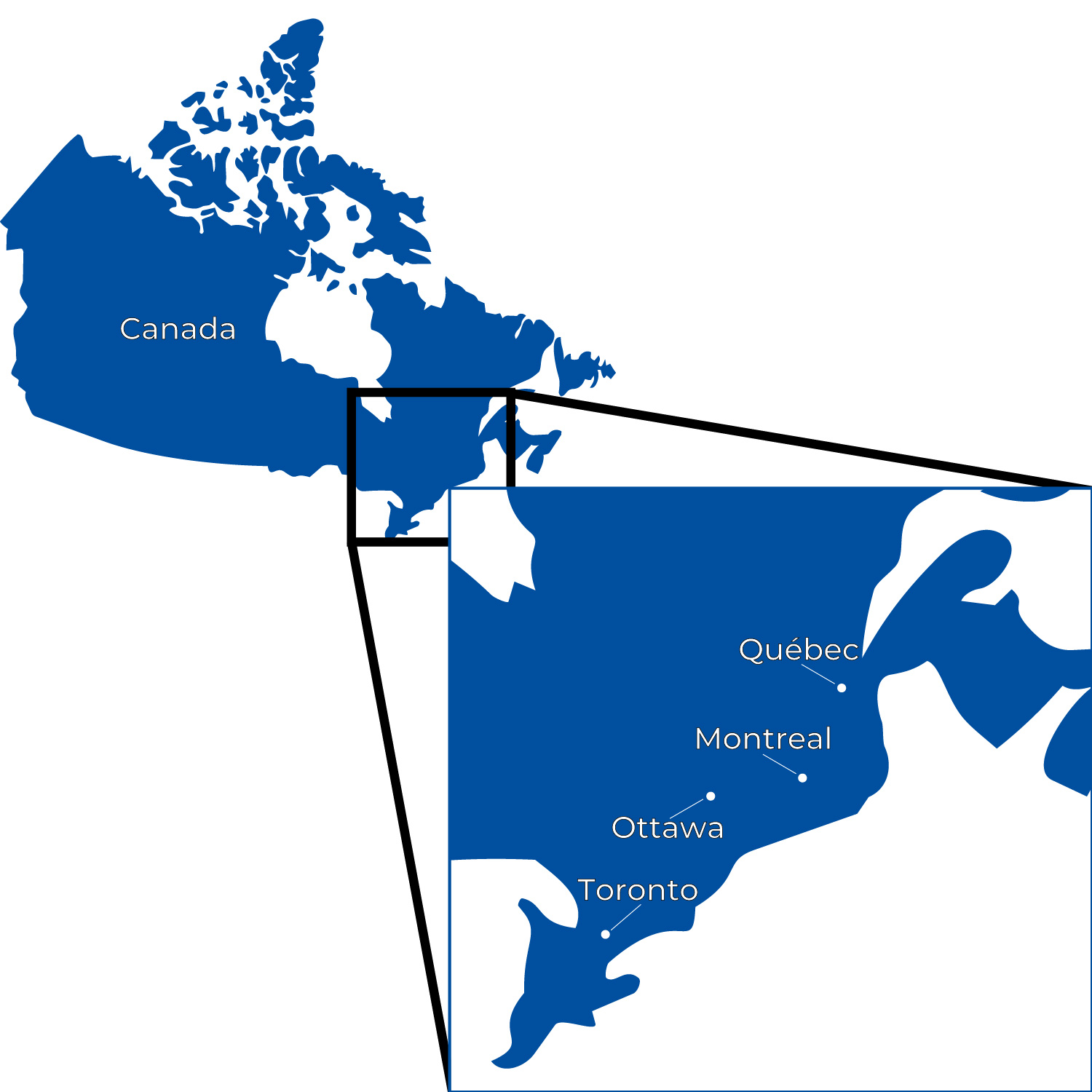
Canada’s life sciences sector has gained significant momentum in recent years. In particular, the Windsor–Toronto–Montreal corridor is increasingly recognized as one of North America’s emerging biotech hubs.
Driven by strong academic institutions, targeted public funding, and growing venture capital activity, the region has developed into a dynamic ecosystem of early- and mid-stage biotech companies.
As part of our international market engagement, ChemCon will be visiting several biotech locations along this corridor in the coming weeks to gain deeper insights into the regional innovation landscape and to foster professional exchange with Canadian life science companies.
Why the Windsor Corridor Is Strategically Relevant
The region combines several structural strengths:
- A high density of research-intensive universities, including the University of Toronto, McGill University, and Western University
- Numerous spin-offs focused on oncology, rare diseases, and specialized small molecules
- Close economic and regulatory alignment with the U.S. market
- International development strategies already established in early phases
For companies advancing innovative APIs into clinical development, the selection of a suitable GMP partner is a critical strategic decision.
Challenges in Early-Stage API Development
Many biotech companies face a pivotal transition:
Moving from laboratory-scale synthesis to GMP-compliant API manufacturing for clinical trials.
Typical requirements include:
- Flexible production of small to mid-scale batches
- High-purity synthesis under strict contamination control
- Comprehensive analytical method development
- Regulatory documentation support (e.g., DMF/ASMF)
- Experience with international regulatory standards, including FDA expectations
While large-scale CDMOs are often optimized for commercial volumes, smaller development facilities may lack full GMP infrastructure. This creates demand for specialized mid-sized manufacturers with strong regulatory expertise.
European GMP Expertise in a Transatlantic Context
As a German CDMO specializing in high-purity APIs and fine chemicals in small to mid-scale quantities, ChemCon has extensive experience supporting clinical development programs.
International inspection experience, robust quality systems, and dedicated production equipment form the basis for reliable collaboration with innovative biotech companies.
In the Canadian market, where development programs are frequently aligned with international regulatory pathways from an early stage, forward-looking manufacturing strategies play a decisive role in minimizing long-term risk.
Dialogue as a Foundation for Successful Development
Our upcoming visit to the Windsor Corridor is intended to strengthen direct professional exchange with the regional biotech community.
Key questions include:
- Which therapeutic areas are currently driving innovation?
- At what development stages are companies operating?
- What specific GMP and analytical requirements are emerging?
- How can transatlantic partnerships be structured efficiently from an early phase?
Open dialogue between drug developers and experienced GMP manufacturers can significantly support scalable and sustainable development strategies.
Connecting with Canadian Biotech Companies
During our visit, we aim to engage with:
- Early- and mid-stage biotech companies
- Developers of niche and orphan drug APIs
- Organizations requiring flexible small-batch GMP manufacturing
- Teams pursuing international regulatory pathways
Outlook
Pharmaceutical innovation is increasingly global. While breakthrough therapies often emerge within regional biotech clusters, specialized GMP manufacturing expertise remains concentrated in experienced production environments.
Active engagement with international innovation ecosystems is therefore an essential element of our long-term strategy.
Through our visit to Canada’s Windsor biotech corridor, ChemCon underlines its commitment to strengthening transatlantic partnerships and supporting innovative API projects with dedicated GMP expertise.
Canadian biotech companies seeking flexible small- to mid-scale GMP manufacturing for clinical-stage APIs are invited to connect with our team during our upcoming visit to discuss potential collaboration opportunities.