Fundamental chemical reactions have often been named after their discoverers/developers. They represent a foundation in organic chemistry and help to set up complicated syntheses. As a contract manufacturer of "small organic molecules" and polymers, ChemCon is naturally involved in the development of syntheses and the transfer of syntheses from the laboratory to a larger scale (upscaling). These naming reactions, all of which have already been applied in ChemCon laboratories, are one of the chemical bases for our synthetic work.
A list of name reactions is given here, which is extended weekly. In this way, a reference work is developing that is not only intended to help students.
Name Reactions:
Osman Achmatowicz was born in Belarus on April 16, 1899. He finished school, including his high school diploma, in Saint Petersburg and studied afterwards at the Stefan Bathory University in Vilnius, where he also belonged to the oldest Polish student fraternity "Konvent Polonia".
He was elected both a member of the Polish Academy of Scholarship and a member of the Warsaw Scientific Society. In 1960, he received an honorary doctorate from the Łódź University of Technology.
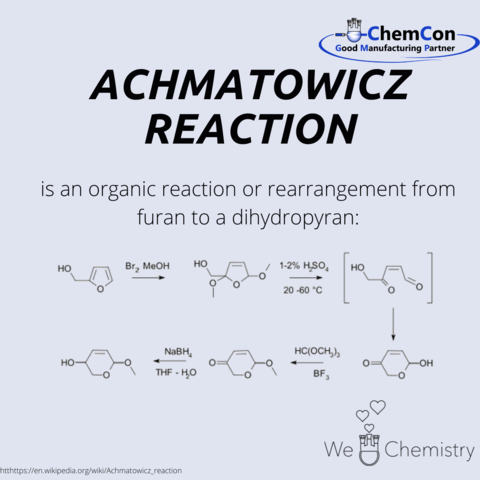
The Achmatowicz reaction developed in 1971 was named after him.
In acyloin condensation, two esters react to form an acyloin. R1 and R2 are organyl radicals.
Mechanistically the 1st ester reacts with sodium, which enables the reaction, forming a radical anion. This anion reacts with the radical anion formed from the other ester to form a dianion. Cleavage of two alcoholate residues (R2O-) forms a diketone, which is reduced with excess sodium to form a dianion. This dianion is then hydrolyzed upon aqueous workup to the α-hydroxyketone, acyloin.
Charles Adolphe Wurtz and Alexander Porfyrech Borodin independently discovered this type of reaction at the end of the 19th century.
Charles Adolphe Wurtz was a French chemist and physician. He was born in Strasbourg in 1817. During his medicine studies, he became very interested in clinical chemistry. Wurtz went to Giessen to work for Justus Liebig for a year, then he returned to Paris. Wurtz was engaged in organic chemistry, especially organic nitrogen compounds. At the famous Sorbonne, Wurtz was the first professor of organic chemistry. The Wurtz-Fittig synthesis is another name reaction in which Charles Adolphe Wurtz collaborated.
Alexander Porfyrech Borodin was a Russian composer, professor of organic chemistry, and physician. Bordi was born in St. Petersburg in the early 19th century. Musical talent and a good musical education made him learn several instruments. In 1850 he began his studies in medicine and in 1859 in chemistry. Already at the age of 29, Borodin received a professorship in organic chemistry. Borodin also conducted research in the field of fluorine compounds.
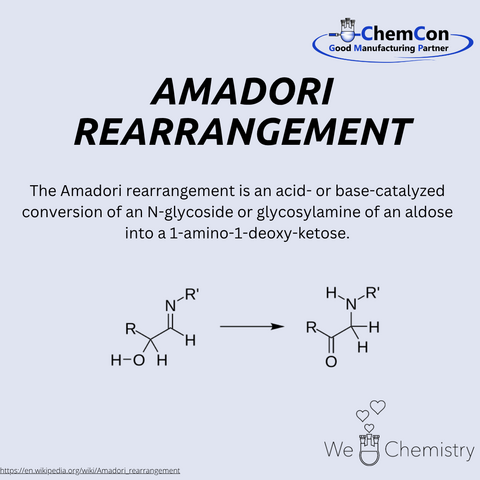
Mario Amadori was an Italian chemist, born on September 18, 1886, in Verona. He worked for many years in both industry and academia, focusing extensively on the chemistry of sugars and amino compounds. He became widely known for discovering the Amadori rearrangement — a key step in non-enzymatic glycosylation. In this process, aldosamines are rearranged under acidic conditions into 1-amino-1-deoxyketoses. The Amadori rearrangement plays a central role in the Maillard reaction, important for flavor formation as well as aging and disease processes. At ChemCon, the Amadori rearrangement is applied in the development of functionalized sugar derivatives and glycoconjugates, which serve as pharmaceutical intermediates and diagnostic building blocks.
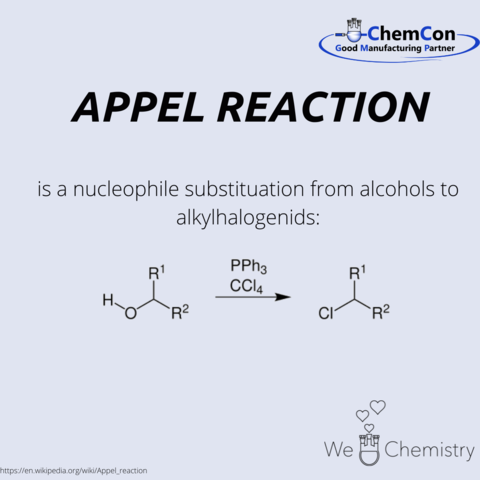
Appel Reaction
Rolf Appel was born in Hamburg in 1921 and studied chemistry at the Martin Luther University Halle-Wittenberg. He received his doctorate in 1951 from the chemist Margot-Becke-Goehring. After graduating, he took over a chemistry chair at the University of Bonn in 1962.
It was a great honor for him to receive the Liebig-Denkmünze in 1986. He has become known for the reaction named after him.
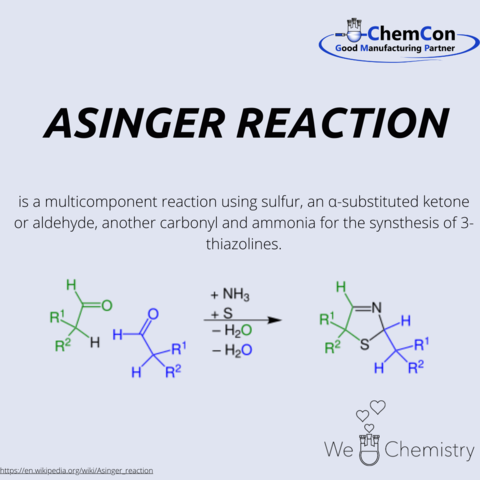
Friedrich Asinger was born in 1907 in Lower Austria. After completing his high school education in Krems an der Donau in 1924, he began his chemistry studies at the Technical University of Vienna. In 1932, he earned his doctorate with a thesis on the influence of substituents on the saponification rate of benzal chloride.
After various industrial positions, Asinger habilitated in 1943 at the University of Graz and became a lecturer at Martin Luther University Halle-Wittenberg in 1944. Due to connections with the NSDAP, he lost this position after the end of World War II. In 1946, he was deported to the Soviet Union with other scientists, where he worked as a group leader in the development of rocket propellants and discovered synthesis pathways for sulfur- and nitrogen-containing heterocycles.
Returning to Germany in 1954, Asinger accepted a call to Martin Luther University Halle (Saale) in 1957, and later to the Technical University of Dresden. During this time, he encouraged, among others, Becker to write the Organikum.
From 1959, Asinger led the Institute of Technical Chemistry and Petrochemistry at RWTH Aachen. Here, he further developed nitrogen-sulfur heterocycle chemistry, now known as Asinger Chemistry. An example is the thirteen-step synthesis of D-penicillamine, with the Asinger Synthesis as the starting reaction.
Through a reaction analogous to the Asinger Synthesis, 3-oxazolines can also be produced. However, the more significant oxazolines are the 2-oxazolines, which can be polymerized through cationic ring-opening polymerization. Depending on the chain length, degree of cross-linking, and attached functional groups, these polymers can exhibit various functionalities. Polymers from 2-oxazoline are termed Polyoxazolines.
ChemCon has been engaged in the synthesis of these polymers for many years and is capable of producing them according to customer specifications under GMP conditions. The company has synthesis and analytics experts and extensive experience in GMP and GMP documentation, as evidenced by inspections from the FDA and German authorities.
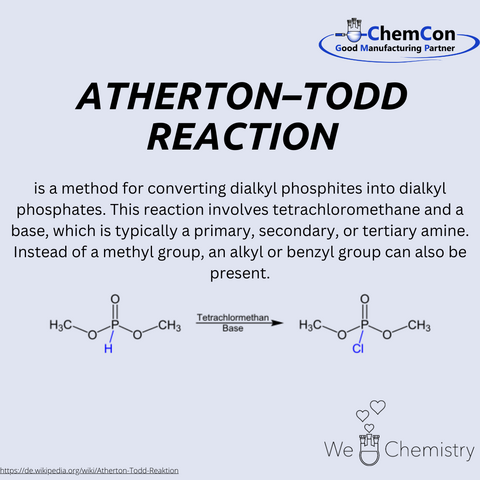
Alexander Roberts Atherton was a distinguished chemist whose contributions to the field have left a lasting impact on modern chemistry. Born in Cambridge, England, in 1911, Atherton's early life was marked by a profound curiosity about the natural world. This curiosity led him to pursue a career in chemistry, where he would go on to make significant strides.
Atherton's academic journey began at the University of Cambridge, where his passion for chemistry quickly became evident. His dedication to his studies earned him numerous accolades, and he graduated at the top of his class with a Bachelor's degree in Chemistry. After completing his undergraduate degree, Atherton pursued a Ph.D. at Goethe University Frankfurt, focusing on organic reaction mechanisms. Following this remarkable achievement, he earned a second Ph.D. at the University of Oxford, where his work centered on the development of new synthetic methods.
One of Atherton's most notable achievements was the development of the Atherton-Todd Reaction, a novel method for converting dialkyl phosphites into dialkyl phosphates. This reaction, which involves the use of trichloromethane and a base, has become a fundamental process in organic synthesis. The versatility and efficiency of the Atherton-Todd Reaction have made it a valuable tool for chemists worldwide and it remains widely used in both academic research and industrial applications.
Atherton's contributions to chemistry were recognized with numerous honors and awards throughout his career. In 1965, he was awarded the Nobel Prize in Chemistry for his groundbreaking work on nucleotides and nucleotide coenzymes. The Nobel Committee highlighted his innovative approach and the broad applicability of his research as significant advancements in the field. In addition to the Nobel Prize, Atherton received several other prestigious awards, including the Copley Medal and the Priestley Medal, both of which are among the highest honors in chemistry.
Atherton's impact extended beyond his research. He was a dedicated educator, mentoring countless students and young scientists who would go on to make their own contributions to the field. His commitment to teaching and his ability to inspire others made him a beloved figure in the academic community.
In his later years, Atherton remained active in the scientific community, frequently attending conferences and collaborating with other leading chemists. His work has been published in numerous scientific journals, and researchers around the world have cited his contributions extensively.
Alexander Roberts Atherton's legacy is one of innovation, dedication, and excellence. His achievements in chemistry have had a profound impact on the field, and his influence will continue to be felt for generations to come. His life serves as an inspiration to aspiring chemists and a reminder of the incredible potential of scientific discovery.
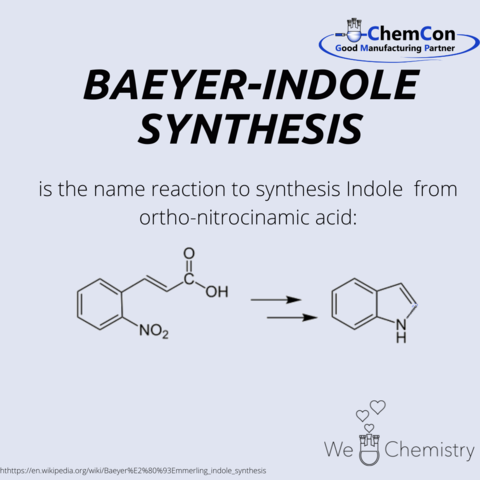
Adolf von Baeyer was born in Berlin in October 1835, the 5th of 7 children. After graduating high school at the Friedrich-Wilhelms-Gymnasium, he studied mathematics and physics at the Friedrich-Wilhelms-University in Berlin and chemistry at the Ruprecht-Karls-University in Heidelberg.
Adolf was a founding member of the "Deutsche Chemische Gesellschaft" (German Chemical Society) in Berlin, which published the technical journal "Berichte der Deutschen Chemischen Gesellschaft" (Reports of the German Chemical Society).
On his 50th birthday, he was raised to hereditary nobility by King Ludwig II of Bavaria and received the title "von".
The Bayer indole synthesis was discovered in 1869 by Adolf von Baeyer and Adolphe Emmerling.
In 1905, von Baeyer won the Nobel Prize for his work on organic dyes.
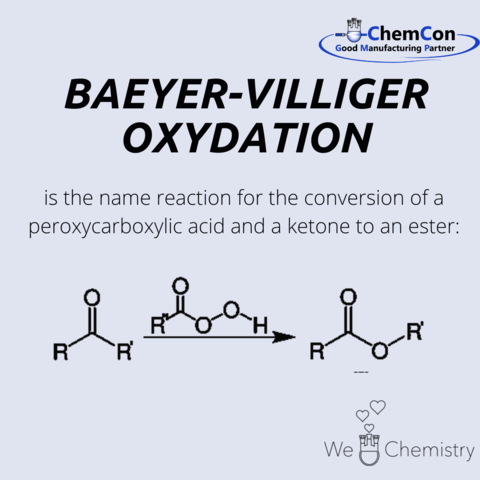
Victor Villiger was born in 1868 on Lake Zug in Switzerland. After leaving school, Villiger studied chemistry at the University of Geneva before being drafted into military service.
In 1890, he transferred to the University of Munich, where he later earned his doctorate with a thesis on Hexahydroisophthalic acid. It was at this time that he met his mentor Adolf von Baeyer at the university. The two worked together for 11 years and jointly developed the Baeyer-Villiger oxidation between 1899 and 1900.
Adolf von Baeyer was born in Berlin in October 1835, the 5th of 7 children. After graduating high school at the Friedrich-Wilhelms-Gymnasium, he studied mathematics and physics at the Friedrich-Wilhelms-University in Berlin and chemistry at the Ruprecht-Karls-University in Heidelberg.
Adolf was a founding member of the "Deutsche Chemische Gesellschaft" (German Chemical Society) in Berlin, which published the technical journal "Berichte der Deutschen Chemischen Gesellschaft" (Reports of the German Chemical Society).
On his 50th birthday, he was raised to hereditary nobility by King Ludwig II of Bavaria and received the title "von".
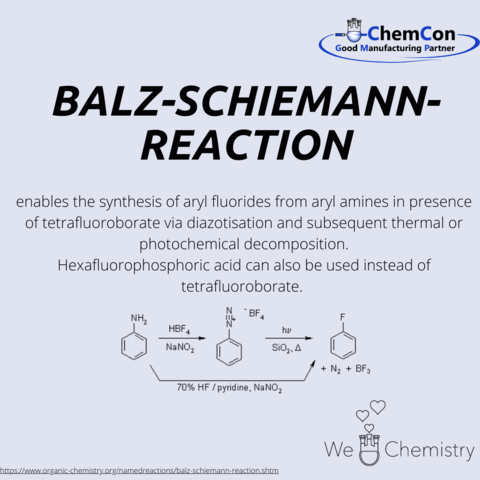
Günther Schiemann was born in Breslau in 1899. He attended the University of Breslau and obtained his Ph.D. in 1925 with a dissertation titled "On the Mechanism of Uric Acid Oxidation." Subsequently, he worked as a volunteer assistant at ETH Zurich under Hermann Staudinger until 1926. In 1926 and 1935, Schiemann served as an assistant and senior assistant at the Technical University of Hannover, where he also taught as a lecturer from 1929. He successfully accomplished the Balz-Schiemann synthesis for the first time in 1927. Due to his Jewish heritage, his employment was terminated in 1935, followed by the revocation of his lecturer position in 1937. Between 1935 and 1950, Schiemann worked in the private sector. In 1946, he became a part-time lecturer in Hannover, and in 1950 a professor at the University of Istanbul, where he headed the "Sinai Kimya Institute." In 1956, he returned as a professor to Hannover and led the Institute of Technical Chemistry there.
Nearly 20% of the 200 best-selling pharmaceutical ingredients of the year 2018 contained at least one aryl fluoride or a derivative thereof.
François Antoine Philippe Barbier, a name not as widely recognized outside the circles of chemistry, holds a pivotal place in the annals of scientific innovation. Born into the rigor of 19th-century France, Barbier's journey into the realm of chemistry was marked by an insatiable curiosity and an unwavering dedication to exploration. His work laid foundational stones for future discoveries, transcending the boundaries of his time and nurturing talents who would further revolutionize the world of science.
Barbier's academic voyage began with a robust education in the sciences, where he displayed remarkable acumen from an early age. His fervor for chemical research propelled him through the ranks of academia, culminating in a distinguished career as a chemist. His scholarly pursuits were characterized by a pioneering spirit, keen to explore the uncharted territories of chemical reactions and their myriad applications.
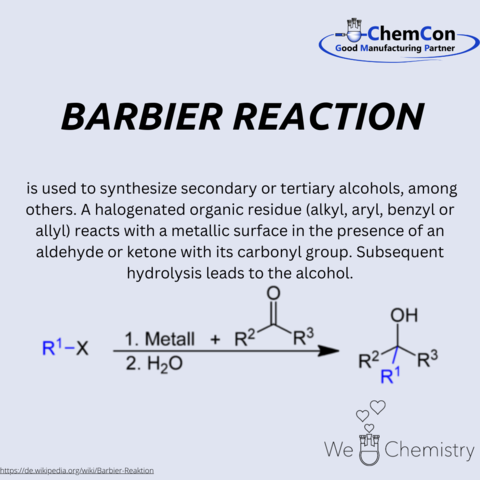
Among Barbier's numerous contributions to chemistry, the Barbier reaction stands as a testament to his ingenuity. This groundbreaking synthesis process, which facilitates the creation of secondary or tertiary alcohols from halogenated compounds, opened new avenues in the synthesis of complex organic molecules. The reaction's elegance lies in its simplicity and efficiency, characteristics that have made it a staple in organic synthesis laboratories around the world.
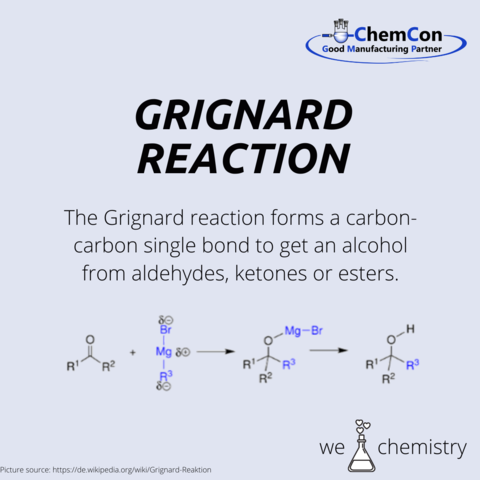
Perhaps one of Barbier's most enduring legacies is his influence on his students, among whom was Victor Grignard, a name synonymous with the Nobel Prize in Chemistry. Grignard, who was awarded the Nobel Prize in 1912, was a direct beneficiary of Barbier's mentorship. The Grignard reaction, an offshoot of Barbier's own research, further expanded the toolbox available to chemists, enabling the formation of carbon-carbon bonds in a manner previously thought to be impractical.
The relationship between Barbier and Grignard exemplifies the profound impact a mentor can have on the trajectory of scientific discovery. It was under Barbier's guidance that Grignard not only honed his skills but also developed the foundational ideas that would lead to his Nobel Prize-winning work. This mentor-mentee dynamic underscores the importance of academic lineage in the propagation of knowledge and innovation.
Barbier's life, filled with academic achievements and scientific breakthroughs, illuminates the path for future generations of chemists. His legacy, characterized by the Barbier reaction and his role in nurturing Nobel laureate talent, continues to resonate within the scientific community. Through his contributions, Barbier not only advanced the field of chemistry but also demonstrated the enduring value of mentorship and the collaborative spirit of scientific inquiry.
As we reflect on the storied career of François Antoine Philippe Barbier, it becomes evident that his work was not just about the molecules and reactions that bore his name but also about fostering a culture of curiosity and perseverance. His legacy, enshrined in the annals of chemistry, serves as a beacon for aspiring scientists, reminding us of the power of exploration and the endless possibilities that await those who dare to question and discover.
Heinrich Wieland, a towering figure in the field of organic chemistry, left an indelible mark on science through his groundbreaking research and influential teaching career. Born on June 4, 1877, in Pforzheim, Germany, Wieland grew up in a family deeply connected to the chemical industry. His early exposure to this environment likely inspired his later passion for chemistry, which he pursued with determination and brilliance.
After completing his studies at the University of Munich, Wieland began his scientific journey with a focus on organic compounds and their intricate mechanisms. Early in his career, he delved into the chemistry of bile acids and nitrogen compounds, areas that would become central to his later accomplishments. Wieland's methodical approach to unraveling complex chemical pathways set him apart as a meticulous and visionary researcher.
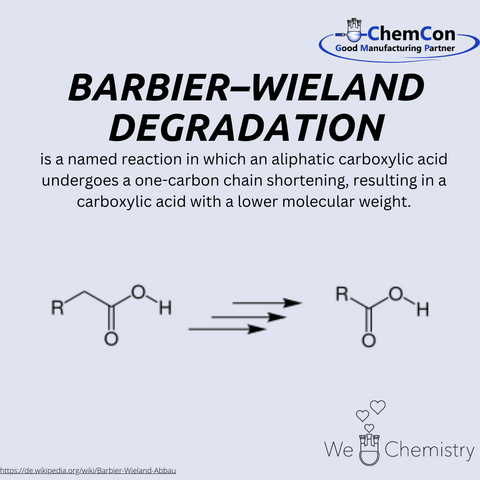
One of his most notable contributions to organic chemistry is the reaction that bears his name: the Barbier-Wieland degradation. This elegant method enables the shortening of carbon chains in aliphatic carboxylic acids by one carbon atom, a technique that became invaluable for structural elucidation in organic synthesis. The reaction reflects Wieland’s ability to design practical methods that expand the tools available to chemists, showcasing his ingenuity and problem-solving skills.
Wieland’s time as a professor at the University of Freiburg from 1913 to 1921 was transformative both for him and for the institution. It was here that he truly honed his skills as a mentor and leader, fostering an environment of intellectual curiosity and rigorous experimentation. Wieland’s lectures were known for their clarity and depth, earning him admiration from students and colleagues alike. Under his guidance, Freiburg became a hub for innovative chemical research, with students drawn to his ability to connect fundamental principles to real-world applications. This period was marked by a vibrant exchange of ideas, as Wieland cultivated a new generation of chemists who would go on to make significant contributions of their own.
In 1927, Heinrich Wieland’s extraordinary achievements were recognized with the Nobel Prize in Chemistry. He was awarded this highest scientific honor for his pioneering research on the constitution and function of bile acids. Wieland's work illuminated the complex biochemistry of these substances, shedding light on their role in digestion and metabolism. The Nobel Committee praised his contributions for advancing the understanding of natural products, an area of chemistry that has profound implications for medicine and biology. Wieland’s Nobel Prize not only affirmed his place among the great chemists of his time but also highlighted the importance of his meticulous and groundbreaking research.
Beyond his scientific accolades, Wieland was deeply committed to fostering a sense of integrity and social responsibility in his academic endeavors. He remained at the forefront of scientific inquiry throughout his career, leaving a legacy that extended far beyond his publications and reactions. Wieland’s life and work exemplify the power of curiosity, determination, and a steadfast commitment to advancing human knowledge.
Heinrich Wieland passed away on August 5, 1957, leaving behind a legacy that continues to inspire generations of chemists. His contributions to science, particularly his research in Freiburg and his Nobel Prize-winning discoveries, remain cornerstones of organic and biochemistry. Through his work, Heinrich Wieland not only advanced the frontiers of chemistry but also set a standard for the profound impact that one individual can have on the scientific world.
Derek H.R. Barton was a British chemist who received the Nobel Prize in Chemistry in 1969. He was born on September 8, 1918, in Gravesend, Kent, and studied at Imperial College of the University of London starting from 1938. After completing his studies, he worked as a chemist in a government program for two years. He then moved to Imperial College in Birmingham, where he worked as a lecturer for two years. From 1946 to 1949, he worked as a research scientist at Imperial Chemical Industries (ICI). His career took a decisive turn when he began his visiting professorship at Harvard University (USA) in the Department of Natural Products Chemistry in 1949. Here he met the US scientist and chemist Robert B. Woodward. Both were connected by a lifelong scientific collaboration and close friendship. This marked the beginning of his groundbreaking scientific work on conformational analysis.
Barton also collaborated with the company Schering-Plough and worked on the topic of “aldosterone” at his research institute for medicine and chemistry in Cambridge. Here he discovered what is now known as the Barton reaction, a photochemical process that enables a relatively simple method for the synthesis of aldosterone. This was a great success of his research work. This led to almost 40 years of close practical relationships between medical research and the pharmaceutical industry.
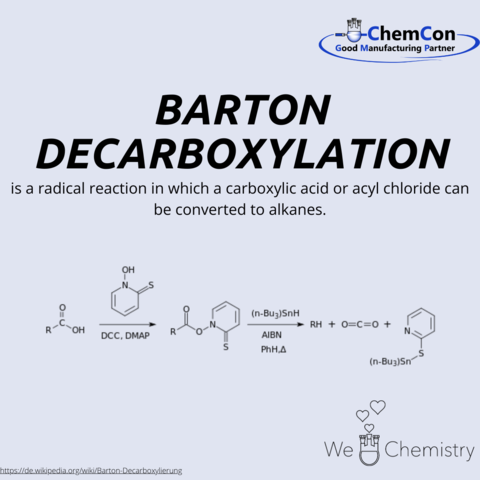
Barton decarboxylation is a name reaction in organic chemistry. It allows organic residues to be cleaved from acid chlorides or carboxylic acids.
Barton’s influence on modern pharmacy is enormous. His research work has contributed to making the synthesis of aldosterone and other steroids easier and more efficient today. His groundbreaking work on conformational analysis has also revolutionized the structure and synthesis of steroids. His discoveries have advanced pharmaceutical research and development and laid the foundation for many important drugs.
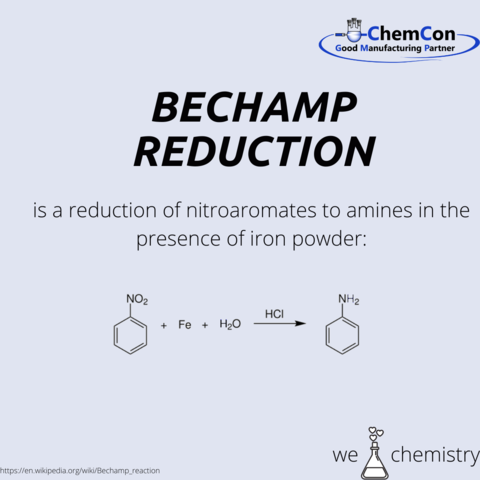
Antoine Béchamp was born in France in 1816, but went to Bucharest with his uncle when he was only seven years old. He began an apprenticeship as a pharmacist, which he finished a few years later in France. After he founded his own pharmacy, he worked also at the pharmacy school in the fields of chemistry, physics and toxicology. During this time he met the chemistry professor Louis Pasteur, to whom he dedicated his doctoral thesis in chemistry. Based on this work, he developed his Béchamp reduction in 1852, which contributed to the rise of the paint industry.
Ernst Otto Beckmann (1853–1923) was a German chemist whose groundbreaking contributions laid a foundation for modern organic and physical chemistry. From his academic pursuits to his innovative techniques and the celebrated Beckmann rearrangement, his legacy remains highly influential in scientific and industrial applications, particularly in the production of essential medications like paracetamol.
Born on May 4, 1853, in Solingen, Germany, Beckmann developed an early interest in science. He initially studied pharmacy and earned his first degree in 1875. His curiosity and ambition soon led him to pursue advanced studies in chemistry at the University of Leipzig under the renowned chemist Hermann Kolbe. Kolbe, a pioneer of structural organic chemistry, greatly influenced Beckmann’s academic development and his later contributions to the field. Beckmann earned his doctorate in 1878, focusing on organic synthesis and the chemistry of amines. His postdoctoral work centered on the structural transformations of organic compounds, setting the stage for his most famous discovery.
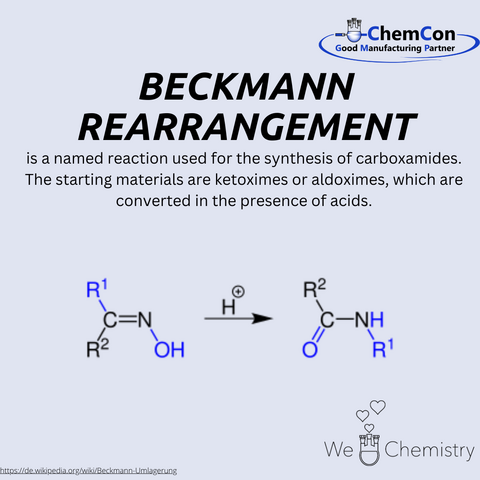
In 1886, Beckmann described a chemical reaction that would bear his name: the Beckmann rearrangement. This reaction involves the acid-catalyzed transformation of ketoximes or aldoximes into carboxamides. The reaction proceeds through the formation of an intermediate species, followed by the migration of a substituent group adjacent to the oxime functionality, ultimately yielding a carboxamide. The versatility of this reaction in converting simple compounds into valuable intermediates made it indispensable in organic synthesis, both in academic research and industrial processes.
The Beckmann rearrangement remains highly relevant today, especially in the pharmaceutical industry. A prominent example is its role in the industrial production of paracetamol (acetaminophen), a widely used analgesic and antipyretic medication. Paracetamol is listed on the World Health Organization's List of Essential Medicines due to its critical role in healthcare worldwide. The reaction enables the efficient and large-scale conversion of chemical precursors into the carboxamide structure that forms the backbone of this life-saving drug, highlighting the enduring significance of Beckmann’s discovery.
In addition to his work in organic chemistry, Beckmann made significant strides in physical chemistry. He developed the Beckmann freezing point apparatus, a device designed to measure the freezing point depression of solutions. This innovation provided a highly accurate method for determining molecular weights and became a standard tool in physical chemistry laboratories. By leveraging the principle that a solvent’s freezing point decreases when a solute is dissolved, Beckmann’s design allowed for precise measurements even in very small temperature ranges, further cementing his reputation as an ingenious experimentalist.
Beckmann’s success was shaped in part by his association with Hermann Kolbe, a towering figure in 19th-century chemistry. Kolbe’s emphasis on systematic structural analysis and rigorous experimental methods deeply influenced Beckmann’s approach to research. Beckmann carried this legacy forward, mentoring students and advancing the field of chemistry through his innovative techniques and discoveries.
Beckmann’s contributions continue to resonate in the modern world. The Beckmann rearrangement, with its enduring utility in synthesizing pharmaceuticals and fine chemicals, underscores the timeless relevance of his work. Paracetamol, one of the most commonly used medicines globally, is an essential component of modern healthcare, treating pain and fever for millions of people daily. Beckmann’s work indirectly ensures access to this critical medication, showcasing how fundamental scientific discoveries can have a profound and lasting impact on public health.
Ernst Otto Beckmann was not just a chemist but an innovator whose discoveries bridged organic and physical chemistry. From his transformative Beckmann rearrangement to his freezing point apparatus, his work exemplifies the profound and lasting influence of scientific inquiry. As we continue to rely on his discoveries for industrial and medical advancements, Beckmann’s legacy serves as a powerful reminder of the enduring power of chemistry to shape our world.
Pietro Biginelli, an Italian chemist born in the late 19th century, is best known for discovering the Biginelli reaction, a pivotal multicomponent chemical process that remains highly relevant in modern pharmaceutical chemistry. Despite limited fame during his lifetime, Biginelli’s work has left a lasting impact on synthetic organic chemistry and the production of active pharmaceutical ingredients (APIs).
Biginelli was born in 1860 and pursued his passion for chemistry at the University of Florence, where he conducted much of his groundbreaking research. Little is documented about his early life, but his academic trajectory led him to become a key figure in chemical synthesis, working on methods to simplify the formation of complex organic compounds. His most renowned discovery, the Biginelli reaction, emerged from his efforts to streamline multi-step processes into a single, more efficient one.
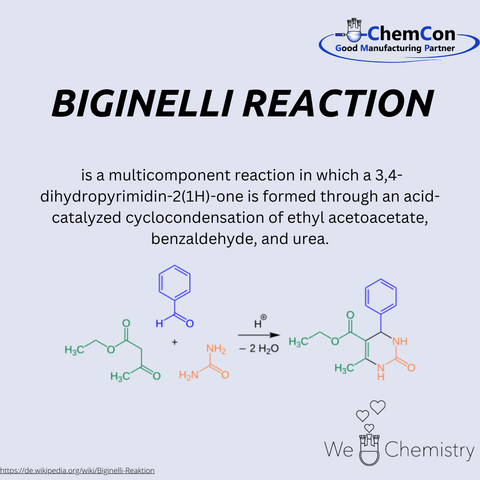
In 1891, Biginelli discovered a reaction that would later bear his name. The Biginelli reaction is a multicomponent cyclocondensation that allows for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones (DHPMs) from simple starting materials: ethyl acetoacetate, benzaldehyde, and urea. The process, catalyzed by an acid, was remarkable for its simplicity and efficiency. At the time, such one-pot reactions were highly sought after because they reduced the number of steps required to create valuable compounds, making the process less labor-intensive and more cost-effective.
Today, the Biginelli reaction plays a crucial role in the synthesis of pharmaceutical compounds, especially APIs. The 3,4-dihydropyrimidin-2(1H)-one core is a common scaffold in biologically active molecules, making it a vital building block in drugs with anti-inflammatory, antiviral, and anticancer properties. The reaction's adaptability, allowing for the incorporation of various substituents, makes it highly valuable in medicinal chemistry for developing new therapeutic agents.
The reaction is widely used in the production of calcium channel blockers, antiviral drugs, and other essential APIs. It remains an essential tool in drug discovery and design, serving as a cornerstone for the development of new pharmaceutical compounds due to its efficiency and versatility.
Though Pietro Biginelli's name might not be as universally recognized as some of his contemporaries, his contribution to chemistry has left a lasting imprint on modern science. The Biginelli reaction continues to facilitate the efficient synthesis of complex molecules and plays an indispensable role in the development of life-saving medications. His discovery exemplifies how curiosity-driven research can have profound and lasting implications for industries like pharmaceuticals, where innovation is constantly evolving. Biginelli’s work serves as a reminder that even modest discoveries can lead to significant advancements, providing foundational tools for critical modern-day applications in healthcare and beyond.
Arthur Birch was born in Sydney, Australia, in 1915. He studied at the University of Sydney, where he received a Bachelor of Science degree in 1937 and a Master of Science degree in 1938. In 1940, he received his doctorate from the University of Oxford/UK. In 1952, Birch accepted a professorship in organic chemistry at the University of Sydney. In 1958, he moved to the UK again to take up a professorship at the University of Manchester. From 1967 to 1980, Birch was dean at the Australian National University of Canberra.
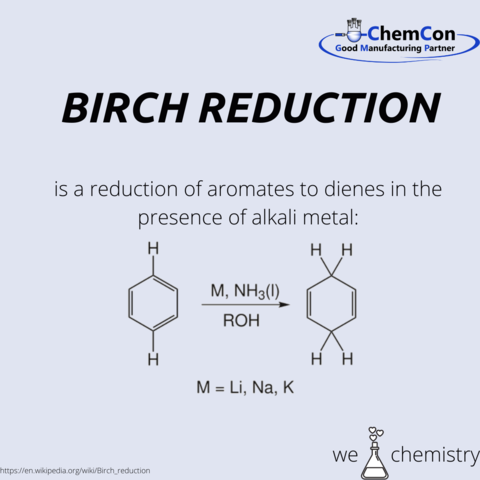
Birch's reduction made it possible to chemically synthesize a steroid for the first time, which is still of great importance to the pharmaceutical industry today.
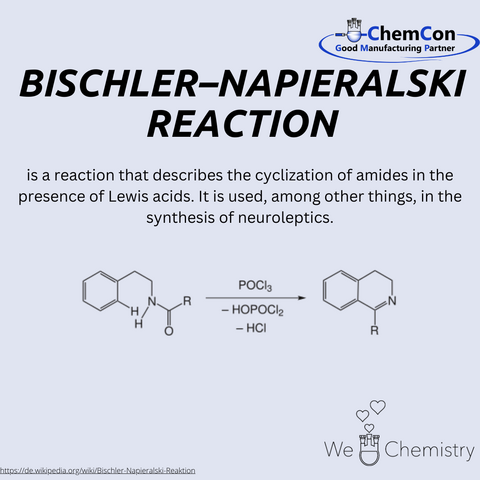
Napieralski is a name that holds significance in the world of chemistry, particularly through his contribution to organic synthesis. Born in the late 19th century, Edmund Napieralski was a chemist whose work has left a lasting impact, most notably through his co-discovery of the Bischler-Napieralski reaction, a key method in the field of heterocyclic chemistry.
While details of his early life and academic background remain scarce, Napieralski’s collaboration with Alfred Bischler, another noted chemist of his time, resulted in the development of a reaction that bears both of their names. This reaction is a cornerstone in the formation of isoquinolines, a class of compounds that are important in the synthesis of alkaloids and pharmaceuticals. The Bischler-Napieralski reaction involves the cyclodehydration of a β-phenylethylamide using a Lewis acid, typically phosphorus pentachloride (PCl5), leading to the formation of isoquinoline derivatives. Isoquinolines are important due to their wide range of biological activities, making this reaction particularly valuable in medicinal chemistry.
One of the prominent applications of the Bischler-Napieralski reaction is its role in the synthesis of neuroleptics, such as loxapine and amoxapine. These compounds are classified as dibenzoxazepine derivatives and are used as antipsychotic medications. Loxapine, for instance, is used to treat schizophrenia and bipolar disorder, while amoxapine is an antidepressant with antipsychotic properties. The underlying structure of these compounds, an isoquinoline ring system, is key to their pharmacological activity, and their synthesis often relies on variations of the Bischler-Napieralski reaction.
Loxapine and amoxapine showcase how a fundamental chemical reaction can be applied to create drugs that address serious mental health conditions. Loxapine, originally synthesized in the 1970s, is known for its dopamine receptor-blocking effects, which help to mitigate symptoms of psychosis. Amoxapine, on the other hand, possesses a unique dual function: it acts as an antidepressant by inhibiting the reuptake of norepinephrine and serotonin, while also having antipsychotic effects due to its interaction with dopamine receptors.
Through the Bischler-Napieralski reaction, Napieralski’s contribution to chemistry extends beyond the lab and into the medical field, where his work plays a vital role in the development of treatments for mental health disorders. His legacy, while not as widely recognized as some of his contemporaries, remains important in both organic chemistry and pharmaceutical research.
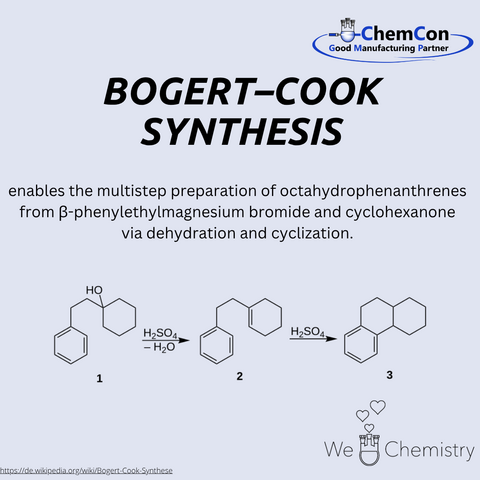
Marston T. Bogert was an American chemist, born on March 18, 1868, in Flushing, New York. He taught for many years at Columbia University and was a leading figure in early American organic chemistry. Together with Alfred W. Cook, he developed the Bogert–Cook synthesis – a method for preparing quinazolinone derivatives via the condensation of anthranilic acid derivatives with amidines. This reaction is relevant in heterocyclic chemistry and is used for producing bioactive compounds. At ChemCon, the Bogert–Cook synthesis is applied in custom synthesis projects, particularly when functionalized quinazolinone scaffolds are required as precursors for pharmaceutical development.
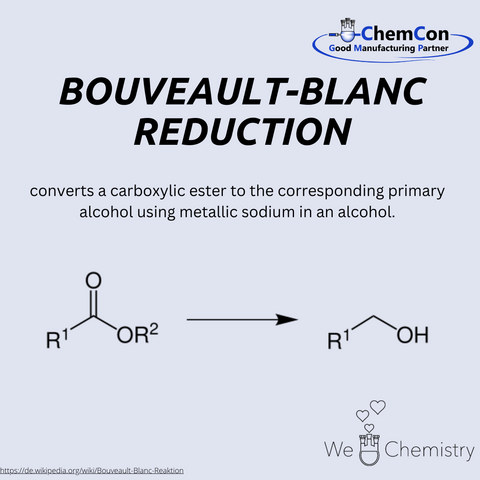
Louis Bouveault was a French chemist, born on February 11, 1864, in Nevers. He taught at several universities, including Paris and Nancy, and focused his research on organic synthesis. Together with the German chemist Georges Blanc, he developed the Bouveault-Blanc reduction, a method for reducing esters to primary alcohols using metallic sodium in alcohol. The reaction was one of the first approaches to directly reduce esters and marked a major advancement in organic chemistry. Although modern alternatives exist today, it remains in use due to its simplicity and effectiveness. At ChemCon, the Bouveault-Blanc reduction is particularly valuable when robust and scalable reduction processes are required, such as in process development.
Adrian Gibbs Brook was a Canadian chemist renowned for his pioneering contributions to organosilicon chemistry. The rearrangement named after him – the Brook rearrangement – describes the intramolecular migration of a silyl group from carbon to oxygen, typically triggered by a base. This reaction allows for the targeted synthesis of silyl ethers and other silicon-containing compounds and is particularly useful in organic synthesis and materials chemistry. At ChemCon, the Brook rearrangement is used in the production of functionalized silicon compounds, especially when they serve as reactive intermediates or protective groups in complex synthetic routes.
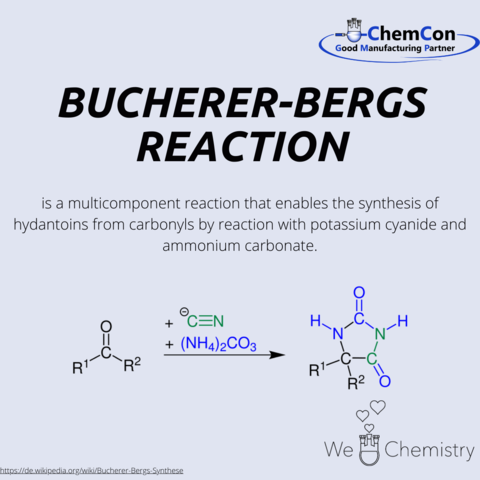
The synthesis named after Hans Theodor Bucherer and Hermann Bergs, and the resulting hydantoins, have numerous practical applications in both the laboratory and industry. Bucherer completed his Abitur in Cologne and studied chemistry at the universities of Munich, Karlsruhe, and Leipzig. He completed his dissertation in 1893 under Johannes Wislicenus in Leipzig, titled 'On some derivatives of keto-hexene from the ketone of α Pimelic acid.' After a period at BASF, he became a private lecturer at the Dresden University of Technology in 1901, and in 1913, he accepted a position at the Berlin Institute of Technology. From 1926, he served as a professor of Chemical Technology at the Technical University of Munich. In addition to his academic roles, he was also active in the chemical industry from 1908 to 1916. Bucherer retired in 1935, and in 1944, he received the Goethe Medal for Art and Science.
In addition to the Bucherer-Bergs synthesis, he also discovered the Bucherer reaction, which allows the conversion of phenols and naphthols, among others, into the corresponding aromatic amines. Hydantoins find applications in various fields: for example in the production of Phenytoin which is used for the long-term treatment of epilepsy and arrhythmias They are also utilized in the manufacturing of sugar derivatives and serve as a precursor for amino acids such as methionine, of which several hundred thousand tons are produced annually.
Hydantoins are derivatives of imidazole. Another derivative is histamine, which can be obtained through the decarboxylation of the amino acid histidine. It is used as histamine dihydrochloride in Ceplene, which is a drug preventing the relapse of acute myeloid leukemia, or as a positive control in allergy tests.
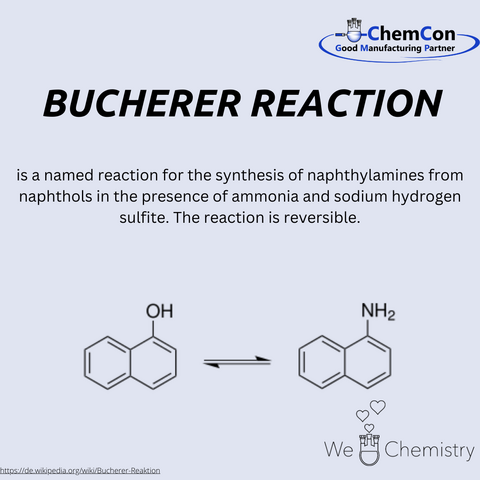
Hans Theodor Bucherer, a name perhaps unfamiliar to many, stands as a cornerstone in the edifice of organic chemistry. Born in 1869, this German chemist carved a significant niche for himself through his academic pursuits and groundbreaking research. His journey, initiated at universities like Munich, Karlsruhe, and Leipzig, culminated in a doctoral degree under the esteemed Johannes Wislicenus. This solid foundation propelled him into a world of academic excellence.
Bucherer's academic trajectory is marked by his ascent to professorships at Dresden, Berlin, and Munich. These roles were not mere titles; they were platforms from which he disseminated knowledge and ignited curiosity. His contributions to the field, however, extend far beyond pedagogy. The chemical landscape bears the imprint of his ingenuity, particularly in the form of the Bucherer reaction. This transformation of naphthols into naphthlamines and vice versa stands as a testament to his experimental prowess.
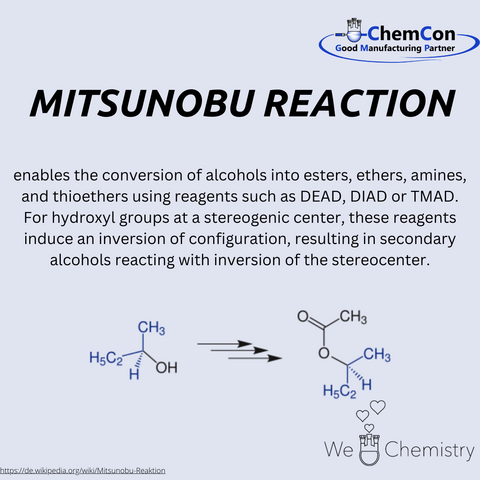
Interestingly, the Bucherer reaction has emerged as a viable alternative to the Mitsunobu reaction. The latter, while effective, often involves toxic reagents and byproducts. In contrast, the Bucherer reaction presents a greener, more environmentally friendly approach. This resurgence of interest in an older reaction underscores the enduring relevance of fundamental research and the potential for rediscovering valuable tools in the chemist's arsenal.
Hans Theodor Bucherer's legacy is a testament to the power of intellectual curiosity and perseverance. His life's work, characterized by the Bucherer reaction and his academic leadership, continues to inspire and influence chemists worldwide. As we navigate the complexities of modern chemistry, it is essential to acknowledge the pioneers who laid the groundwork, and Bucherer undoubtedly occupies a prominent place in this pantheon of scientific heroes.
John F. Hartwig is a renowned American chemist known for his pioneering work in the field of metal-catalyzed reactions. Born in the United States, he developed an early interest in chemistry, which led him to an impressive academic career. He earned his bachelor's degree in chemistry from Princeton University and completed his PhD at the University of California, Berkeley. Afterward, he conducted postdoctoral research at the Massachusetts Institute of Technology (MIT) before accepting a professorship at Yale University. He later moved to the University of Illinois and is currently a professor at the University of California, Berkeley.
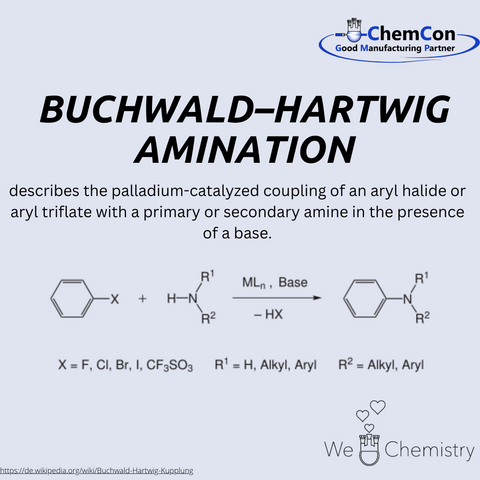
His research focuses on developing new catalytic processes, particularly in the area of transition metal catalysis. One of his most significant achievements is the Buchwald-Hartwig amination, developed in collaboration with Stephen Buchwald. This reaction describes the palladium-catalyzed coupling of aryl halides or aryl triflates with primary or secondary amines in the presence of a base. This method has proven to be highly efficient for synthesizing arylamines, which play a central role in organic chemistry.
The Buchwald-Hartwig amination has had a profound impact on organic chemistry and, in particular, the pharmaceutical industry. Before its development, the synthesis of arylamines was often inefficient, labor-intensive, and costly. The introduction of this palladium-catalyzed method made it possible to produce a wide range of complex amines under mild reaction conditions with high yields. These advancements facilitated the synthesis of many pharmaceutically relevant molecules, as arylamines are present in numerous active pharmaceutical ingredients. Today, the Buchwald-Hartwig amination is an indispensable tool in drug research and production and is used worldwide in academic and industrial laboratories.
ChemCon also utilizes the Buchwald-Hartwig amination as an integral part of the production of pharmaceutical active ingredients and intermediates. The flexibility and efficiency of this reaction enable ChemCon to produce complex molecules with high purity while adhering to strict GMP (Good Manufacturing Practice) guidelines. This is essential to meet the high-quality standards of the pharmaceutical industry. By applying this modern coupling technology, ChemCon can offer customized solutions and develop innovative active pharmaceutical ingredients efficiently.
For his outstanding contributions to chemistry, John F. Hartwig has received numerous awards, including the American Chemical Society Award in Organometallic Chemistry and the Wolf Prize in Chemistry. His influence on the scientific community and the chemical industry is immense. His work forms an essential foundation for future developments in metal-catalyzed chemistry. Hartwig's research has not only expanded the understanding of fundamental chemical processes but also enabled new applications that have a lasting impact on modern drug development.
Ludwig Rainer Claisen born January 14th 1851 in Cologne was a German chemist. He graduated school in 1869 in Cologne and studied chemistry in Bonn and Göttingen until 1871. Under Kekulé he finished his PhD in 1875 with a thesis about “Beiträge zur Kenntniss des Mesityloxyds und des Phorons“ (Contributions to the knowledge of the Mesityloxyd and the Phoron) and started being Kekulés assistant.
Three years later he habilitated and worked as a private lecturer. After some time at the Owens College in Manchester and in Munich he heeded the call to Aachen University, where he became ordinary for the chemistry department. In 1897 he moved to Kiel University where he retired in 1902 for health reasons. He did two more years of research in Berlin before he started his own laboratory.
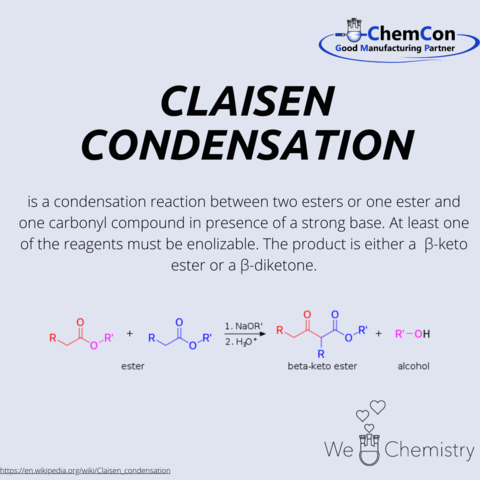
He was a member of the “Bavarian Academy of Science” as well as an honorary member of the “German chemical society”. In 1881 he was elected as part of the German National Academy of Sciences Leopoldina. In the same year he discovered the “Claisen-condensation”. There is also some laboratory equipment named after him the “Claisenbrücke” and the “Claisenkolben”.
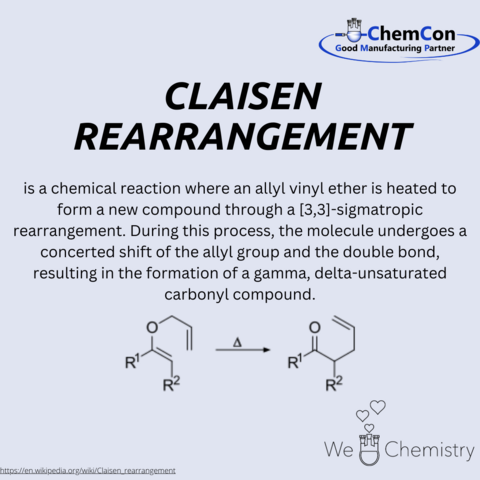
Ludwig Claisen was a German chemist born on January 14, 1851, in Cologne. He is best known for developing the Claisen rearrangement, a fundamental organic reaction that bears his name. Claisen studied chemistry at the University of Bonn and later at the University of Göttingen, where he completed his doctorate under the supervision of Friedrich Wöhler, one of the pioneers of organic chemistry.
Throughout his academic career, Claisen made significant contributions to the field of organic chemistry, including his work on ester condensation reactions—now known as Claisen condensations—which are crucial for forming carbon-carbon bonds in various synthetic procedures. His research greatly expanded the toolkit of synthetic organic chemistry, influencing the development of pharmaceuticals, agrochemicals, and new materials.
Claisen held positions at several prestigious institutions, including the University of Berlin and the University of Kiel. His work laid foundational principles that have been pivotal in the advancement of synthetic methodologies in modern chemistry. The reactions he discovered and the techniques he developed continue to be integral in the synthesis of complex molecular architectures in academic and industrial settings worldwide. His legacy is not only marked by the reactions named after him but also by his role in training the next generation of chemists during his tenure as a professor.
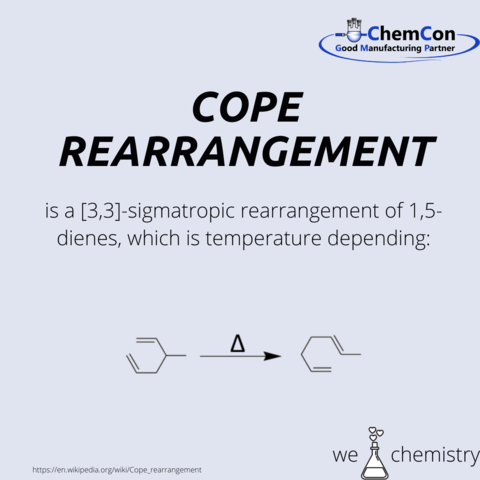
Arthur C. Cope
...was a US-American chemist and professor of organic chemistry at the Massachusetts Institute of Technology (MIT) in Cambridge.
He received his PhD with the topic “The synthesis of local anesthetics containing various phenylalkyl groups: Vinylethyl malonic ester and the cleavage of certain substituted malonic esters with sodium ethoxide” at the University of Wisconsin–Madison in 1932.
During World War II, he conducted a series of researches for chemical weapons, anti-malaria compounds and the treatment of mustard gas victims.
At the MIT, he headed the chemistry department starting in 1945. The preparative organic chemistry was one of his fields of work, especially elimination and condensation reactions. Due to this the cope rearrangement, Diaza Cope rearrangement and Cope elimination were named after him.
Elias James Corey was born on July 12, 1928, in Methuen, Massachusetts, and became one of the most influential chemists of the 20th century. From an early age, he showed a strong interest in science and mathematics, leading him to study at the Massachusetts Institute of Technology (MIT). He earned his PhD at just 22 years old and embarked on an academic career that led him to Harvard University in 1959, where he remained for decades, shaping the field of organic chemistry. His most significant contribution to science was the development of retrosynthetic analysis, a method that allows chemists to design complex molecules by breaking them down into simpler building blocks. This groundbreaking technique revolutionized organic synthesis and became a standard strategy in modern chemistry. Corey's analytical approach enabled chemists worldwide to devise efficient and precise synthetic routes for a wide range of molecules. For his work, Corey received numerous awards, including the Nobel Prize in Chemistry in 1990. In addition to the Nobel Prize, he was honored with prestigious awards such as the National Medal of Science and the Priestley Medal.
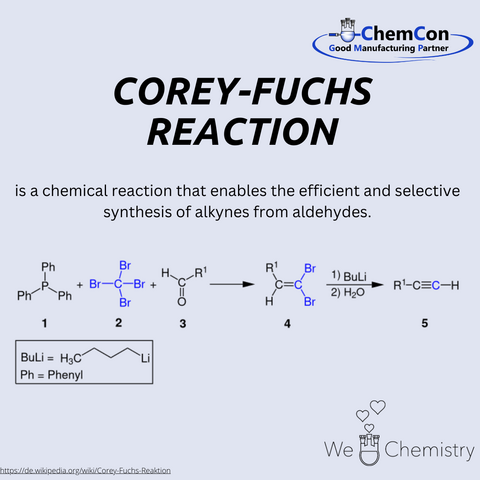
One specific contribution by Corey to synthetic chemistry is the Corey-Fuchs reaction, a method for synthesizing alkynes from aldehydes. This reaction involves converting aldehydes into dibromides using triphenylphosphine and carbon tetrabromide, followed by treatment with strong bases to yield terminal alkynes. This approach is particularly useful for efficiently producing terminal alkynes with high precision. The reaction finds widespread use in pharmaceutical chemistry and the synthesis of complex organic molecules. ChemCon applies such established methods in custom synthesis to develop tailored solutions for pharmaceutical applications. By integrating these reactions into process development, ChemCon delivers high-purity and specification-compliant compounds for research and industrial partners. The ability to efficiently implement complex synthesis routes makes ChemCon a valuable partner for companies seeking highly specialized chemical compounds.
Elias James Corey: A Chemist Who Changed the World
Elias James Corey was born on July 12, 1928, in Methuen, Massachusetts. He is an American chemist and Nobel laureate. Corey is an emeritus professor at Harvard University. His research over five decades spans nearly all areas of organic chemistry and has had a profound impact on biochemistry and modern medical science.
Academic Career
Corey received his bachelor's degree in chemistry from Harvard University in 1950. He then earned his Ph.D. at the University of Illinois at Urbana-Champaign under John C. Sheehan. After his Ph.D., Corey worked as a postdoctoral fellow at the Massachusetts Institute of Technology (MIT) under Robert Burns Woodward.
In 1954, Corey returned to Harvard University, where he was awarded a professorship in organic chemistry. He remained at Harvard University until his retirement in 2000.
Scientific Achievements
Corey has made numerous significant contributions to organic chemistry throughout his career. His most notable achievements include:
- The development of new synthesis methods for complex natural products, including steroids, terpenes, and alkaloids.
- The investigation of the mechanisms of organic reactions.
- The development of new molecular catalysts.
Corey's work has helped to deepen our understanding of organic reactions and open new pathways for the synthesis of complex molecules. It has also had important implications for biochemistry and modern medical science.
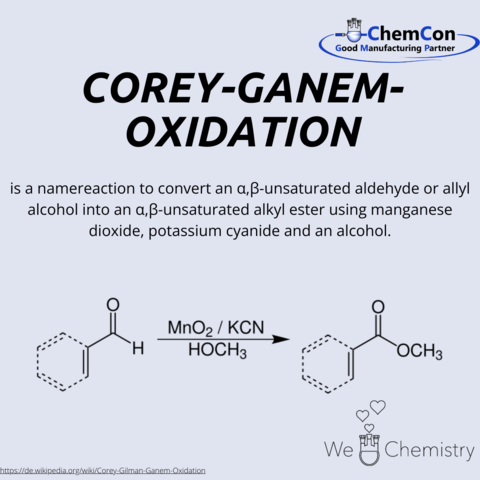
Corey-Ghanem Oxidation
The Corey-Ghanem oxidation is an important synthesis method for the oxidation of alcohols to ketones or aldehydes. The reaction was developed in 1967 by Elias James Corey and his student Samir A. Ghanem.
The Corey-Ghanem oxidation proceeds in two steps. In the first step, the alcohol is oxidized with a strong oxidizing agent, such as chromium trioxide. In the second step, the resulting ketone or aldehyde is reduced with a reducing agent, such as ammonia.
The Corey-Ghanem oxidation is a versatile and efficient synthesis method that can be applied to a variety of alcohols. It is particularly useful for the oxidation of sensitive alcohols that are not stable with other oxidation methods.
Awards
Corey has received numerous awards for his scientific achievements throughout his career. His most notable awards include:
- The Nobel Prize in Chemistry in 1990
- The National Medal of Science in 1985
- The Copley Medal of the Royal Society in 1998
Corey is one of the most important chemists of the 20th century. His work has revolutionized organic chemistry and has had important implications for biochemistry and modern medical science.
Elias James Corey is an American chemist who was awarded the Nobel Prize in Chemistry in 1990 for his development of the theory and methodology of organic synthesis, particularly for his establishment of retrosynthesis.
Corey was born in Methuen, Massachusetts, in 1928, to Lebanese Christian parents. He studied chemistry at the Massachusetts Institute of Technology, where he earned his bachelor's degree in 1948 and his doctorate in 1951. From 1951 to 1959, he served as a professor at the University of Illinois, where he became a full professor at the age of 27. Since 1959, he has been a professor at Harvard University.
Throughout more than five decades, Corey conducted research in nearly all areas of organic chemistry and made significant contributions to biochemistry and modern medical science. He developed numerous synthesis methods and reagents, such as pyridinium chlorochromate and 1,3-dithiane as a protecting group for carbonyl compounds. He also explored organometallic chemistry, catalytic asymmetric reactions, and provided mechanistic insights into bond formation. He was the first to use computers for designing synthetic routes.
One of his most notable contributions is retrosynthesis, a logical method for planning the synthesis of complex organic molecules from simpler precursors. Retrosynthesis is based on the principle that a complex structure can be broken down into simpler parts by imagining how it could be formed through known reactions. These parts can then be further deconstructed until reaching readily available starting materials. Retrosynthesis is a powerful tool for the synthesis of natural products, pharmaceuticals, and other biologically active compounds.
Corey conducted several hundred total syntheses of natural products, including prostaglandins, steroids, antibiotics, and alkaloids. He also synthesized the antiviral drug Oseltamivir (Tamiflu), effective against influenza.
Corey is one of the most prolific and influential chemists of all time. He has published over 1000 scientific papers and received more than 70 scientific awards. Additionally, he has trained numerous industrial chemists as well as future professors and Nobel laureates, such as Ryoji Noyori and Bengt Ingemar Samuelsson.
Theodor Curtius, a distinguished German chemist whose name is synonymous with several key developments in chemistry, was born in Duisburg, situated in the Ruhr industrial region of Germany. His journey into the world of chemistry began at some of Germany's finest institutions, studying under the guidance of Robert Bunsen at Heidelberg University and Hermann Kolbe at Leipzig University, where he received his doctorate in 1882.
His professional path was marked by significant appointments and contributions. From 1884 to 1886, he worked under Adolf von Baeyer at the University of Munich, an experience that honed his research skills and prepared him for future academic leadership roles. He then directed the analytical chemistry department at the University of Erlangen until 1889, demonstrating his proficiency and dedication to the field.
Curtius's career continued to ascend as he accepted the chair in Chemistry at the University of Kiel. During his tenure, he was recognized for his productivity and research acumen, earning him the appointment as Geheimer Regierungsrat (Privy Councillor) in 1895. His achievements led him to Bonn University in 1897, where he filled the prestigious role previously held by August Kekulé. The following year, he returned to his alma mater, Heidelberg University, succeeding Victor Meyer as Professor of Chemistry. He remained a central figure there until his retirement in 1926, shaping the minds and careers of future chemists.
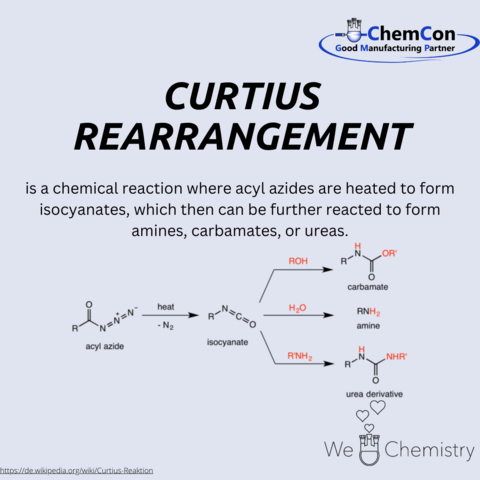
One of his most notable scientific contributions is the Curtius rearrangement, a significant reaction in organic chemistry that involves the thermal decomposition of acyl azides into isocyanates, which are key intermediates in the synthesis of amines, carbamates, and ureas. This reaction has broad applications in pharmaceuticals and polymer sciences, showcasing Curtius's lasting impact on chemical synthesis.
Outside the laboratory, Curtius was equally passionate about music and the outdoors. He was an avid mountaineer and founder of the Kiel section of the Association of German and Austrian Alpinists in 1894, a group he supported generously. His musical talents were displayed in his compositions and performances at concerts, adding layers to his persona beyond his scientific achievements. During his time in Munich, he developed a close friendship with Christian Klucker, a renowned alpinist guide, with whom he embarked on numerous mountaineering adventures.
Theodor Curtius's life and work were marked by a blend of rigorous scientific inquiry and a deep appreciation for the arts and nature. He passed away in Heidelberg on February 8, 1928, leaving behind a legacy that continues to influence the fields of chemistry and beyond. His contributions are remembered not only through his scientific discoveries but also through his personal passions, which made him a well-rounded scholar and individual. His life is a testament to the impact one can have bridging the gap between science and the humanities.
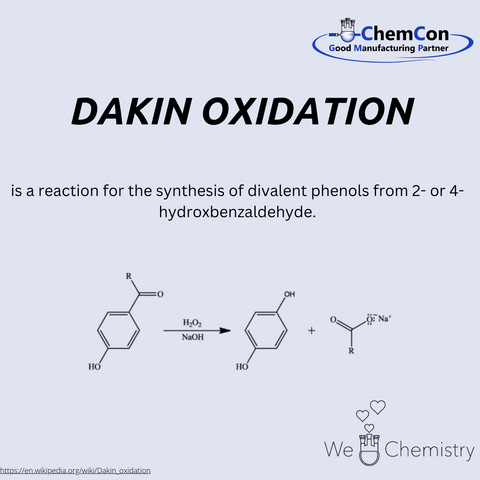
Henry Drysdale Dakin was a British chemist, born on March 12, 1880, in London. He studied at Emanuel College, Cambridge, and later conducted research in the United States, including at Columbia University. Dakin was a pioneer in the field of biochemical oxidation processes and collaborated with prominent medical researchers. He is best known for developing the Dakin oxidation, a reaction that converts aromatic aldehydes into phenols. This oxidation takes place under mild conditions using hydrogen peroxide in an alkaline medium. The method is particularly selective and gentle, making it attractive even today for transforming sensitive molecules. At ChemCon, the Dakin oxidation is employed to selectively modify structures while preserving sensitive substituents – a clear advantage in the synthesis of pharmaceutical intermediates.
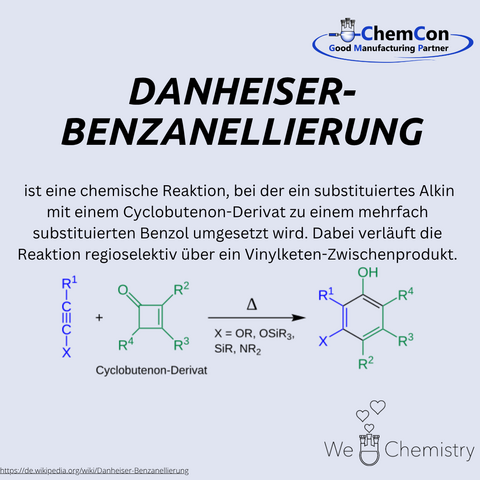
Rick L. Danheiser is an American chemist and professor at the Massachusetts Institute of Technology (MIT), where he has long been a leading figure in the field of organic synthesis. He is especially known for his work on new methods in aromatic chemistry. Most notably, the Danheiser benzanellation is a reaction that allows the formation of substituted aromatic systems via electrophilic cyclizations from functionalized precursors. This approach enables the construction of complex polycyclic aromatic structures and is particularly valuable in natural product synthesis and drug discovery. At ChemCon, the Danheiser benzanellation is used to construct aromatic ring systems with precision, especially when demanding substitution patterns must be implemented.
Otto Paul Hermann Diels and Kurt Alder:
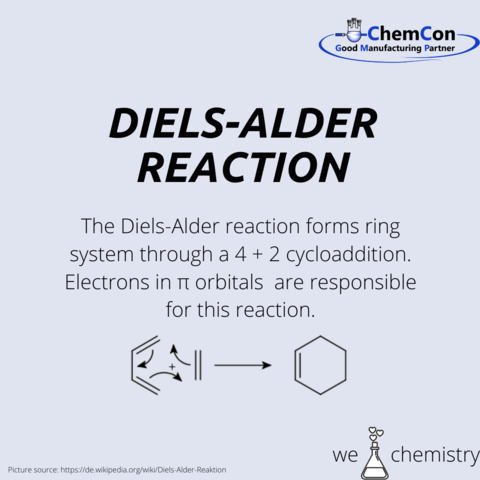
Diels was born in Hamburg and moved with his family to Berlin, where he studied chemistry. He remained at the University of Berlin until 1915, when he accepted a position at the University of Kiel, where he remained until his retirement in 1945. It was during his time at Kiel, where he worked with Kurt Alder developing the Diels–Alder reaction.
Alder was born in the industrial area of Königshütte, Silesia. When Königshütte became a port of Poland he moved to Berlin. He made his PhD in Kiel where he met Mr. Diels. Alder received several honorary degrees and other awards, such as the 1950 Nobel Prize in Chemistry, which he shared with his teacher Diels for their work on the Diels–Alder reaction.
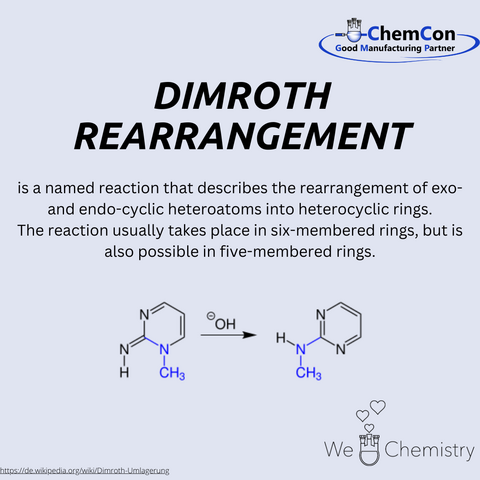
Otto Dimroth, born on March 28, 1872, in Bayreuth, Germany, is celebrated as a significant figure in organic chemistry. His contributions to the field are varied and impactful, with the Dimroth rearrangement and the Dimroth condenser standing out as two of his most notable innovations.
Dimroth's educational journey took him to the University of Munich and the University of Erlangen, where he was mentored by prominent chemists. This early exposure to advanced chemical synthesis and reaction mechanisms fueled his passion and laid a strong foundation for his future research. His academic career saw him holding professorships at several German universities, including the University of Jena and the University of Würzburg. Throughout his tenure, Dimroth's meticulous approach to chemical synthesis and his deep understanding of reaction mechanisms were evident in his work.
Among his many contributions, the development of the Dimroth rearrangement stands out. This reaction involves the migration of substituents within a molecule, particularly within 1,2,3-triazoles, and has broad applications in the synthesis of complex organic compounds. The rearrangement can be summarized as the conversion of a substituted 1,2,3-triazole into another substituted 1,2,3-triazole, facilitated by strong bases or heat. This reaction is especially valuable in heterocyclic chemistry, playing a crucial role in the production of various nitrogen-containing compounds used in pharmaceuticals and agrochemicals.
Another significant invention by Dimroth is the Dimroth condenser, also known as the Dimroth cooler. This piece of apparatus is used in distillation processes and is particularly effective for reflux applications. Its unique design, featuring a coiled inner tube through which cooling water flows, surrounded by vapors to be condensed, provides a larger surface area for heat exchange. This makes the Dimroth condenser highly efficient and reliable, and it has become a staple in chemical laboratories worldwide, especially in organic synthesis where precise temperature control is essential.
Otto Dimroth's contributions to chemistry extend far beyond his eponymous reactions and apparatus. His work has influenced generations of chemists, facilitating advances in both academic research and industrial applications. The principles underlying the Dimroth rearrangement continue to be explored and applied in modern chemical synthesis, demonstrating the enduring relevance of his discoveries.
Dimroth passed away on May 16, 1940, but his legacy lives on through the many chemists who continue to build upon his foundational work. His life and career serve as an inspiration, highlighting the importance of curiosity, dedication, and innovation in scientific endeavors. From his early days as a student in Germany to his influential roles as a professor and researcher, Otto Dimroth's profound scientific inquiry and achievements have left an indelible mark on the field of chemistry. His development of the Dimroth rearrangement and the Dimroth condenser are testaments to his ingenuity, cementing his place in the annals of scientific history. As the complexities of chemical reactions and processes continue to be explored, Otto Dimroth's work remains a guiding light, reminding us of the power of human ingenuity in unlocking the mysteries of the natural world.
Karl Heinz Dötz was a remarkable figure in the field of organic chemistry, known for his pioneering contributions that have left a lasting impact on modern chemical synthesis. Born in Germany in 1943, Dötz embarked on a scientific journey that would see him become one of the most respected chemists of his time.
Dötz's academic career began with his studies in chemistry, where he displayed an early aptitude for complex problem-solving and innovation. He pursued his doctoral studies under the guidance of some of the leading chemists of the era, where he developed a strong foundation in organic chemistry. His work during this period set the stage for what would become a groundbreaking contribution to the field: the Dötz reaction.
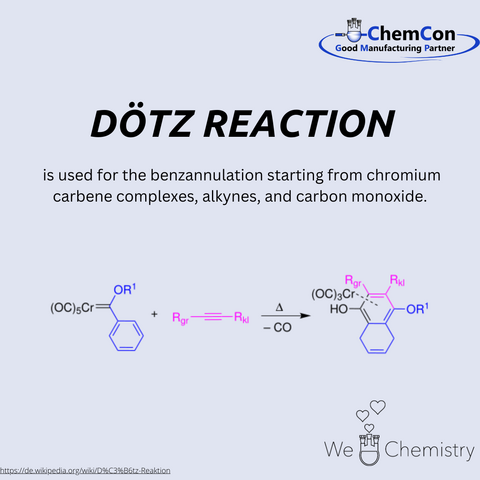
The Dötz reaction, also known as the benzannulation reaction, is a method of synthesizing aromatic compounds from chromium carbene complexes, alkynes, and carbon monoxide. This reaction was first reported by Dötz in the late 1970s and quickly garnered attention for its elegance and utility. The process involves the formation of a highly reactive chromium carbene intermediate, which then reacts with an alkyne and carbon monoxide to produce a substituted phenol or aromatic ring. The reaction is highly regioselective and offers a direct route to complex aromatic structures that are often difficult to obtain through other means.
One of the reasons the Dötz reaction has stood the test of time is its versatility. It provides chemists with a powerful tool to construct benzene rings, which are fundamental building blocks in organic chemistry. Aromatic compounds are ubiquitous in nature and are found in a wide range of natural products, pharmaceuticals, and materials. The ability to efficiently synthesize these structures has made the Dötz reaction invaluable in various fields of research and industry.
Today, the Dötz reaction is still widely used, particularly in the synthesis of natural products and complex organic molecules. Its application extends to the pharmaceutical industry, where it is employed in the creation of drug candidates and other biologically active compounds. The reaction's efficiency and selectivity make it an attractive choice for chemists looking to create intricate molecular architectures with precision.
Karl Heinz Dötz's legacy extends beyond his eponymous reaction. Throughout his career, he made numerous contributions to the field of organometallic chemistry, mentoring a generation of chemists who have gone on to make their own significant contributions. His work exemplifies the power of innovative thinking in science, where a single reaction can open up new avenues of research and application.
In conclusion, Karl Heinz Dötz's life and work have had a profound impact on the field of chemistry. The Dötz reaction remains a cornerstone of modern synthetic chemistry, and its continued use today is a testament to the ingenuity and foresight of its creator. As we look to the future, the principles underlying the Dötz reaction will undoubtedly continue to inspire new discoveries and advancements in the chemical sciences.
Emil Erlenmeyer Jr. emerged from the shadows of a remarkable lineage, carrying forward the legacy of his father, Emil Erlenmeyer Sr., a luminary in the world of chemistry. Born into an environment rich with scientific discourse and innovation, Erlenmeyer Jr. was naturally inclined toward the sciences, particularly chemistry, which he pursued with fervor and dedication.
Erlenmeyer Jr. received his education at some of Europe's finest institutions, developing a deep understanding of chemical processes. His academic path was heavily influenced by his father’s achievements and methodologies. Emil Erlenmeyer Sr., renowned for inventing the Erlenmeyer flask in 1860, was a pivotal figure in chemistry during the 19th century. His contributions extended beyond this iconic piece of laboratory equipment; he was instrumental in the development of the theory of structure in organic chemistry, which posited that the arrangement of atoms within a molecule determines its properties.
One of Emil Jr.'s significant contributions to the field was the "Erlenmeyer Synthesis," a process that involves the formation of β-hydroxy ketones from α-diazoketones. This reaction highlighted his ability to leverage complex organic chemistry techniques to facilitate new synthesis processes, which have had long-standing implications for the manufacturing of various organic compounds, particularly in pharmaceuticals.
The influence of his father was unmistakable in Erlenmeyer Jr.'s career. Emil Sr.’s teachings not only provided a solid foundation in the basic principles of chemistry but also instilled a sense of curiosity and a drive to innovate. This paternal influence was evident as Emil Jr. expanded on his father’s scientific ideas, helping to transition from traditional chemical practices to more modern approaches that have become standard in today’s scientific and medical research environments.
Emil Erlenmeyer Sr.'s role as a pioneer is reflected in his contributions to organic chemistry, particularly through his work on the structure and behavior of various organic compounds. His research paved the way for the acceptance and expansion of structural theory in organic chemistry, which has fundamentally shaped the way chemists understand and manipulate molecules today.
Through his own innovations and contributions, Emil Erlenmeyer Jr. not only honored his father's legacy but also helped forge a link between past and contemporary chemical practices. His work continues to influence modern chemistry, particularly through methods and synthesis techniques that have become essential in the development of new drugs and treatments. In this way, both Emil Erlenmeyer Sr. and Jr. have left a lasting impact on the field, illustrating how the torch of scientific inquiry can be successfully passed from one generation to the next, each building upon the foundation laid by the last.
Albert Eschenmoser was one of the most significant chemists of the 20th century, leaving behind an impressive scientific legacy. Born in 1925 in Switzerland, he began his academic career at ETH Zurich, where he later became a professor. His research focused on organic chemistry, particularly in the synthesis and structural elucidation of complex natural products. He made groundbreaking contributions to the total synthesis of vitamin B12, one of the most challenging projects in organic chemistry, which he successfully completed together with Robert Burns Woodward. This achievement made him known worldwide and earned him numerous scientific awards, including the Copley Medal of the Royal Society and the Wolf Prize in Chemistry.
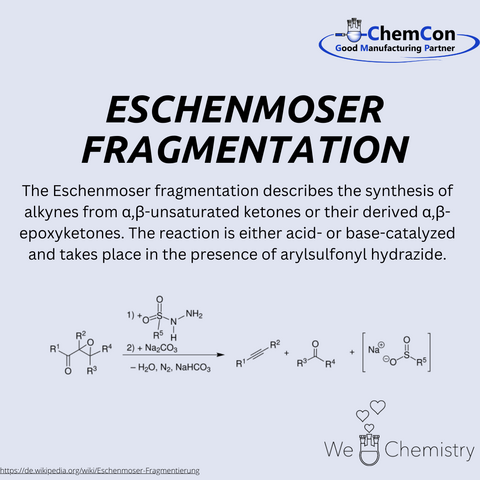
In addition to the vitamin B12 synthesis, Eschenmoser explored the chemical foundations of life. His work on prebiotic chemistry significantly contributed to the understanding of the possible origins of nucleic acids. He investigated alternative nucleic acid structures and developed the concept of Eschenmoser fragmentation, a reaction that became highly significant in organic synthesis. His deep understanding of natural products and their biosynthesis had a far-reaching impact on pharmaceutical research and the development of new active compounds.
Even today, his findings remain highly relevant to modern medicine. The synthetic production of natural products makes it possible to develop highly effective drugs based on natural models. This is where ChemCon comes in: As a specialist in contract manufacturing and custom synthesis, ChemCon synthetically develops natural products to make them available for research and industry. Especially in the pharmaceutical sector, tailored natural product derivatives are essential for drug development. ChemCon’s expertise in GMP-compliant contract manufacturing ensures that customers receive highly pure and reliable substances that meet the highest regulatory standards.
Albert Eschenmoser’s research continues to influence modern organic chemistry and the development of new medicines. His work demonstrates how essential the connection between fundamental research and applied science is—a principle that ChemCon also follows to provide innovative solutions for the pharmaceutical and biotechnological industries. The ability to synthesize natural products precisely opens new paths in drug discovery and highlights the importance of chemical synthesis for the medicine of the future.
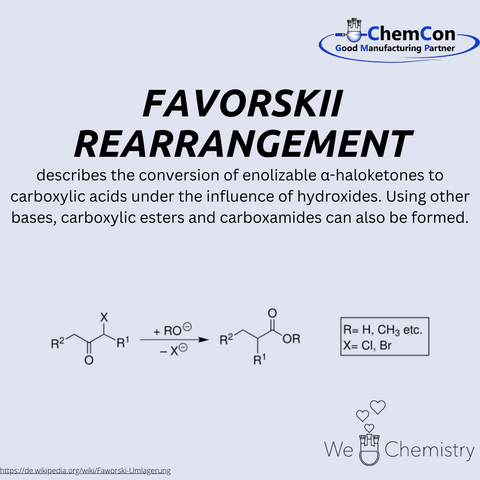
Alexei Yevgrafovich Favorsky, a prominent Russian chemist, was born on March 14, 1860, and passed away on August 8, 1945. Favorsky made significant contributions to the field of organic chemistry, particularly through the discovery and study of the Favorskii rearrangement, which is named after him.
Favorsky began his scientific career at the University of St. Petersburg, where he later became a professor. His work focused on the mechanisms of organic reactions and the synthesis of complex organic compounds. The Favorskii rearrangement, a reaction he discovered, is a remarkable transformation in which enolizable α-haloketones are converted into carboxylic acids when treated with hydroxides. Interestingly, using different bases allows the synthesis of carboxylic esters and amides. The reaction proceeds via the formation of an enolate, followed by an intramolecular rearrangement and the hydrolysis of an intermediate cyclopropanone.
A significant application of the Favorskii rearrangement is in the synthesis of cubanes. Cubane, a compound with the chemical formula C₈H₈, is characterized by its unusual structure where all carbon-carbon bond angles are 90°. This structure imparts cubane with unique physical and chemical properties. The first synthesis of cubane, carried out by Philip Eaton and Thomas Cole in 1964, involves several steps, with the Favorskii rearrangement playing a crucial role in the complex synthetic pathway.
Cubanes are of great interest due to their unique structure and high strain energy. The right-angled bond geometry, which is extremely rare in nature, results in compounds with high density and stability. These properties make cubanes particularly useful in various applications, including medicine and material science. For instance, cubanes are used in the development of high-energy fuels and explosives, as well as in pharmacology as stable scaffold structures for creating new drugs.
Through his research and discoveries, Alexei Yevgrafovich Favorsky has left a lasting impact on organic chemistry. His work on the Favorskii rearrangement has not only provided fundamental insights into reaction mechanisms but also enabled significant applications in the synthesis of complex molecules, including the remarkable cubane compound. His creative and innovative approach to chemical problems remains an inspiring legacy in the world of chemistry.
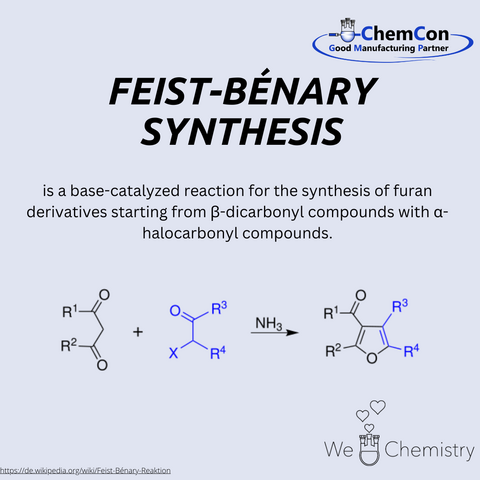
Franz Feist was a German chemist of the early 20th century, born on May 1, 1869. He earned his doctorate from the University of Strasbourg and worked in the fields of carbohydrate and dye chemistry. His name is primarily associated today with the Feist-Benary synthesis, which he developed together with Ernst Benary. This reaction enables the synthesis of furan derivatives from α-haloketones and β-keto esters under basic conditions. The method is of great interest in pharmaceutical and heterocyclic chemistry due to its efficiency in producing functionalized furans. At ChemCon, the Feist-Benary synthesis is employed in custom syntheses to selectively produce heterocyclic compounds for research or as intermediates for more complex drug molecules.
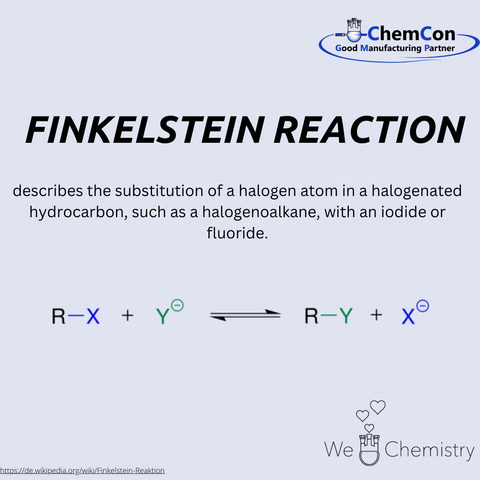
Hans Finkelstein is a significant figure in the history of chemistry, particularly in organic halogen chemistry. Born in the late 19th century, the German chemist dedicated himself to the study of halogen exchange reactions and left a legacy with the Finkelstein reaction, which remains relevant in the chemical industry today.
Finkelstein studied chemistry at a renowned German university, where he developed an early interest in organic synthesis methods. His primary research focus was on the substitution of halogen atoms in organic compounds. Particularly remarkable was his discovery that chlorine or bromine substituents in organic molecules could be efficiently replaced by iodide using sodium iodide in acetone. This insight led to the development of the Finkelstein reaction, which is regarded as an elegant method for halogen exchange in organic synthesis.
The Finkelstein reaction is a nucleophilic substitution reaction (SN2) in which one halogen atom is replaced by another. It plays a crucial role in the production of organic iodides, which serve as key raw materials in pharmaceutical synthesis, agrochemistry, and materials science. The reaction's simplicity and high selectivity make it a standard procedure in chemical research and production.
Hans Finkelstein’s legacy continues to influence the chemical and pharmaceutical industries today. Many modern synthesis methods are based on his principles or were inspired by his discoveries. Halogen exchange reactions remain highly relevant in the production of active ingredients and specialty chemicals.
ChemCon GmbH carries on the legacy of precise and high-quality synthesis processes, supporting the chemical and pharmaceutical industries with tailored solutions. As a specialist in contract development & manufacturing, ChemCon offers innovative approaches to the production of high-purity active ingredients, intermediates, and specialty chemicals. With extensive expertise in synthesis development, the company enables customized synthesis pathways for demanding projects—from laboratory development to GMP production.
By combining classical organic chemistry with state-of-the-art technology, ChemCon remains a reliable partner for the pharmaceutical and chemical sectors. The principles once established by Hans Finkelstein through his research continue to live on in ChemCon’s innovative developments and tailored synthesis strategies.
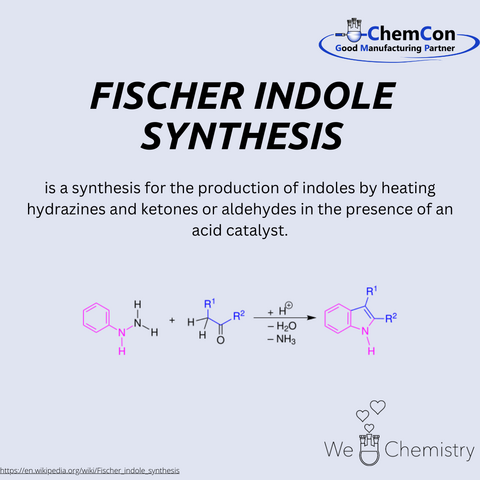
Emil Fischer, a towering figure in the realm of chemistry, was born on October 9, 1852, in Euskirchen, Germany. His early years were shaped by a strong educational foundation, thanks to his father, a successful businessman, who instilled in him the values of discipline and curiosity. Fischer's academic journey began at the University of Bonn, where he initially studied chemistry. However, it was his transfer to the University of Strasbourg that truly marked the start of his illustrious career. There, under the mentorship of Adolf von Baeyer, Fischer's passion for organic chemistry flourished.
Fischer's doctoral work, completed in 1874, focused on phenolphthalein and laid the groundwork for his future endeavors. After earning his PhD, he briefly worked at the University of Munich before accepting a position at the University of Erlangen. It was here that Fischer began to make significant strides in his research on purines and sugars. His meticulous studies on the structure and synthesis of these compounds earned him widespread recognition and eventually led to his appointment at the University of Würzburg in 1888, and later at the University of Berlin in 1892.
One of Fischer's most remarkable achievements was his work on the stereochemistry of sugars and purines. His pioneering research on the structure of glucose and other sugars revolutionized the field of carbohydrate chemistry. By devising the Fischer projection, a method to represent the three-dimensional structure of molecules on a two-dimensional plane, he provided chemists with a powerful tool to visualize and understand the spatial arrangement of atoms within a molecule. This breakthrough not only elucidated the structures of many sugars but also paved the way for advancements in biochemistry and molecular biology.
In addition to his contributions to carbohydrate chemistry, Fischer's work on purines was equally groundbreaking. He identified the structure of uric acid and caffeine, and his synthesis of various purines laid the foundation for the development of pharmaceuticals and the understanding of nucleic acids, the building blocks of DNA and RNA. His work in this area was so impactful that it earned him the Nobel Prize in Chemistry in 1902.
Beyond his scientific achievements, Fischer was known for his dedication to teaching and mentorship. He guided numerous students who went on to become prominent chemists in their own right. Fischer's ability to inspire and cultivate talent was a testament to his passion for chemistry and his commitment to advancing the field.
Despite facing personal challenges, including the loss of his first wife and struggles with his health, Fischer remained steadfast in his scientific pursuits until his death on July 15, 1919. His legacy is preserved not only in the numerous awards and honors he received but also in the enduring impact of his work on modern science.
Emil Fischer's life and career exemplify the relentless pursuit of knowledge and the profound impact that one individual can have on the scientific community. His contributions continue to resonate, influencing contemporary research and inspiring future generations of chemists.
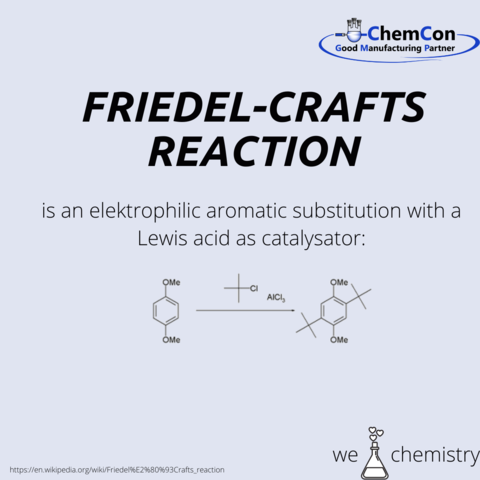
1850 Friedel studied science in Strasbourg and, after an interruption; he continued his studies at Sorbonne in 1852. From 1856 to 1870, he worked as curator of the mineral collection of the École des Mines. During this time, he deepened his chemical knowledge under Charles Adolphe Wurtz in the laboratory of the École de médecine. In 1861, Charles Friedel and James Mason Crafts met here. After James earned a Bachelor of Science degree (1858), further studies took him to the Freiberg Mining Academy in 1859, to the University of Heidelberg in 1860 and to the École de Médecine in Paris in 1861. In 1877, they discovered the catalytic effect of aluminum chloride in reactions of aromatics with alkyl halides, today known as Friedel-Crafts reactions.This is a fundamental reaction that every chemist learns and which is also carried out in the ChemCon laboratories.
Tohru Fukuyama is a distinguished figure in the realm of organic chemistry, celebrated for his pioneering research and the development of innovative synthetic methods, most notably the Fukuyama amine synthesis. This method revolutionized the synthesis of secondary amines, providing a novel pathway through nosyl amides and employing thiolate-induced elimination to achieve this transformation. Beyond its technical merits, the Fukuyama amine synthesis has broadened the horizons for the synthesis of polyamides and has been instrumental in enhancing solid-phase synthesis techniques. Solid-phase synthesis can be used to produce bio polymers. Fukuyama's academic journey is marked by numerous achievements, including impactful publications and the mentorship of the next generation of chemists. His work has significantly contributed to the advancement of pharmaceuticals and materials science, showcasing his profound influence on both academic research and practical applications in chemical synthesis.
Sigmund Gabriel was born in Berlin on November 7th 1851. He studied and completed his doctorate in Heidelberg under Robert Wilhelm Bunsen until 1874. He was a professor at Berlin University until 1921, where he investigated organic nitrogen compounds. In 1887, he developed the Gabriel synthesis named after him for the synthesis of primary alkylamines. In 1888, he was also elected a member of the German National Academy of Sciences Leopoldina.
The reaction is a pure laboratory process due to poor atom economy. The synthesis of primary amines from haloalkanes and ammonia is not possible as amines of other alkylation stages are formed. One example of a secondary amine is piperidine, which was first isolated by the Danish chemist Hans Christian Ørsted.
Victor Grignard:
Failed entrance exams for mathematics – Served in the military - Nobel Prize winner for Chemistry
Although he initially failed the entrance exam, he tried again after a year in the military and was successful. This was not enough for him and he switched to chemistry.
Due to Dr. Grignard, who received the Nobel Prize for Chemistry in 1912 (together with Mr. Paul Sabatier), it is nowadays possible to perform syntheses with advanced methods in organic chemistry.
Grignard published around 170 scientific articles about his work and worked on a large chemical encyclopedia in French until his death.
Even if he faced a challenge at the beginning, he never gave up.
Arthur Rudolf Hantzsch, born on March 7, 1857, in Dresden, Germany, was a trailblazing chemist whose contributions to organic chemistry continue to shape the field today. His innovative methodologies, groundbreaking theories, and impact on both academic and applied sciences have cemented his legacy in the history of chemistry.
Hantzsch was raised in a period of significant scientific progress, which likely influenced his passion for chemistry. He pursued his studies at the Dresden Polytechnic and later at the University of Würzburg, where he was mentored by the prominent chemist Johannes Wislicenus. His academic journey culminated in a doctorate, setting the stage for a prolific career in research and education.
Hantzsch's early work focused on the reactivity and synthesis of nitrogen-containing compounds. His research into pyridines, a class of heterocyclic aromatic compounds, led to the development of the Hantzsch-Widman system. This nomenclature system, co-developed with Otto Widman, provides a systematic way to name heterocyclic compounds based on ring size and constituent elements. The system remains a cornerstone in chemical nomenclature, facilitating clear and consistent communication in scientific literature.
One of Hantzsch's most famous contributions is the Hantzsch dihydropyridine synthesis, a reaction that he first described in 1881. This method involves the condensation of a β-keto carbonyl compound, an aldehyde, and ammonia (or a primary aliphatic amine) to produce 1,4-dihydropyridines. These compounds are precursors to pyridines and have significant applications in medicinal chemistry.
Today, the Hantzsch synthesis is celebrated for its utility in producing calcium channel blockers, a class of drugs essential for treating cardiovascular diseases like hypertension and angina. This direct link to the pharmaceutical industry underscores Hantzsch's enduring influence on modern medicine.
Hantzsch's work earned him numerous accolades during his lifetime. He held prestigious academic positions, including professorships at the University of Würzburg, Leipzig, and the University of Zurich. His election to various scientific academies and receipt of honorary degrees reflect the high regard in which he was held by his contemporaries.
Hantzsch's research laid foundational principles in organic synthesis, influencing both theoretical and applied chemistry. His contributions to heterocyclic chemistry have been instrumental in the development of numerous pharmaceuticals. The Hantzsch dihydropyridine synthesis, for instance, paved the way for the discovery of life-saving drugs such as nifedipine, amlodipine, and other dihydropyridine-based calcium channel blockers.
Moreover, his systematic approach to chemical nomenclature through the Hantzsch-Widman system has enabled chemists worldwide to describe complex molecules with precision and clarity, fostering collaboration and innovation across disciplines.
Arthur Hantzsch passed away on March 14, 1935, but his legacy endures. His pioneering methods and systems continue to be taught in chemistry courses and utilized in research labs across the globe. Hantzsch’s work exemplifies the profound impact that a single scientist can have, bridging the gap between theoretical exploration and practical application.
As we reflect on his contributions, it is clear that Hantzsch was more than a chemist; he was a visionary who foresaw the potential of his discoveries to transform science and society. His influence will undoubtedly inspire future generations of chemists to push the boundaries of knowledge, just as he did over a century ago.
Richard F. Heck, an American chemist and Nobel laureate, is celebrated for his groundbreaking contributions to organic chemistry. His development of the Heck Reaction, a palladium-catalyzed coupling process, revolutionized synthetic chemistry and became a cornerstone for pharmaceutical development and material science. This post delves into his life, academic journey, and the lasting impact of his work, emphasizing its relevance to industries like Chemcon’s.
Richard F. Heck was born on August 15, 1931, in Springfield, Massachusetts. His early fascination with science and technology led him to pursue a Bachelor’s degree in Chemistry at the University of California, Los Angeles (UCLA), followed by a Ph.D. in 1954 under the guidance of Saul Winstein. Heck's doctoral research laid the foundation for his later focus on organometallic chemistry.
After completing his Ph.D., Heck conducted postdoctoral research at the ETH Zurich, one of the world’s premier institutions in chemistry. He later joined Hercules Powder Company in Delaware, where he began exploring palladium chemistry. This work set the stage for his tenure at the University of Delaware, where his most significant achievements unfolded.
In 1971, Heck published his seminal work on the palladium-catalyzed coupling of aryl halides with olefins, now known as the Heck Reaction. This reaction allows for the formation of carbon-carbon bonds with remarkable precision and efficiency. Its versatility and broad applicability have made it an indispensable tool in modern organic synthesis. The Heck Reaction’s utility lies in its ability to streamline the synthesis of complex organic molecules, enable the production of pharmaceuticals including anticancer and antiviral drugs, and facilitate the creation of advanced materials such as polymers and liquid crystals.
The Heck Reaction’s impact on chemistry and industry cannot be overstated. It is widely employed in drug development, including the synthesis of active pharmaceutical ingredients (APIs). The reaction’s efficiency aligns with the principles of green chemistry, minimizing waste and reducing the environmental footprint of chemical manufacturing. For companies like Chemcon, which specialize in GMP-grade chemicals, the Heck Reaction underscores the importance of high-purity reagents and catalysts. Its role in streamlining pharmaceutical synthesis makes it a model of innovation and sustainability.
In 2010, Richard F. Heck, along with Akira Suzuki and Ei-ichi Negishi, received the Nobel Prize in Chemistry for “palladium-catalyzed cross couplings in organic synthesis.” This accolade highlighted the transformative nature of his work, which continues to inspire chemists worldwide. Heck’s legacy extends beyond his scientific achievements. His commitment to pushing the boundaries of chemistry serves as a reminder of the importance of innovation and collaboration in solving complex challenges.
Richard F. Heck’s pioneering work has left an indelible mark on chemistry, particularly in the pharmaceutical and materials industries. The Heck Reaction exemplifies how fundamental research can drive technological advancements and create pathways for sustainable manufacturing. For Chemcon and similar companies, Heck’s contributions highlight the critical role of high-quality reagents and innovative processes in achieving excellence in chemical production. His legacy inspires ongoing innovation, ensuring that the chemical industry continues to meet the demands of a rapidly evolving world.
August Wilhelm Hofmann (from 1888 "von Hofmann") was born in Giessen in 1818. He matriculated in 1836 at what is now Justus Liebig University in Giessen; initially for law, but later for chemistry under Justus Liebig. In 1841 he received his doctorate under Liebig with a thesis on the "Chemical Investigation of Organic Bases in Coal Tar ".
In 1845, the "Royal College of Chemistry" in London was founded with Hofmann as its first director. After the death of Prince Albert, the support of the British industry decreased; so Hofmann decided to accept a call to Berlin University as a professor. He taught inorganic and organic chemistry, wrote a textbook and developed the Hofmann voltameter.
Together with Nobel Prize winner Adolf Bayer, among others, he founded the "Deutsche Chemische Gesellschaft" (German Chemical Society); the predecessor association to the "Gesellschaft Deutscher Chemiker" (GDCh).
The Hofmann elimination is a relevant mechanism in anesthesiology with regard to the inactivation of certain muscle relaxants.
Rolf Huisgen, born on June 13, 1920, in Gerolstein, Germany, is a distinguished German chemist renowned for his groundbreaking work in organic chemistry. His academic journey began at the University of Bonn, where he pursued chemistry, inspired by the innovative environment and his professors. After completing his doctoral studies under the guidance of Nobel laureate Otto Diels in 1943, Huisgen continued to make significant strides in his field.
Following his doctorate, Huisgen held various academic positions, ultimately becoming a professor at the University of Munich in 1952. His tenure there marked a period of prolific research and numerous contributions to organic chemistry. Huisgen's most notable achievement is the development of the 1,3-dipolar cycloaddition reaction, commonly known as the Huisgen cycloaddition. This reaction is a cornerstone in the synthesis of five-membered heterocycles, fundamentally altering the landscape of synthetic chemistry.
Among his many scientific accomplishments, the Huisgen pyrrole synthesis stands out. This method involves the reaction of an α-amino acid with a carboxylic acid and an alkyne to form a substituted pyrrole. The pyrrole ring system is a crucial component in many natural products and pharmaceuticals, making this synthesis invaluable for drug discovery and development. Huisgen's innovative approach to cycloaddition reactions has paved the way for advancements in materials science, medicinal chemistry, and chemical biology.
Throughout his career, Rolf Huisgen has received numerous accolades, including the prestigious Adolf von Baeyer Medal and the Order of Merit of the Federal Republic of Germany. His contributions have left an indelible mark on the field of organic chemistry, inspiring countless researchers and shaping modern synthetic methods. Rolf Huisgen's legacy continues to influence the scientific community, demonstrating the profound impact of his work on contemporary chemistry.
The Hunsdiecker reaction is named after Heinz Hunsdiecker and his wife Cläre, who further developed the method in the 1930s. Heinz Hunsdiecker was a German chemist specializing in organic halogenation. The reaction builds on earlier work by Alexander Borodin and Carl Ruff. It involves the reaction of silver carboxylates with halogens – typically bromine – to form alkyl halides with loss of CO₂. This decarboxylative halogenation is particularly useful for shortening carbon chains. At ChemCon, the Hunsdiecker reaction is applied in synthesis projects where targeted chain shortening or halogenation is required, such as in the modification of intermediates for pharmaceutical compounds.
James Irvin significantly contributed to chemistry, notably through the Irvine-Purdie methylation technique. His journey in chemistry began with a strong academic foundation, leading him to pursue higher education and eventually a PhD. It was during his postgraduate studies that he, alongside his colleague Purdie, developed this methylation method, which has since become a valuable tool in synthetic chemistry.
The technique itself is acclaimed for its efficiency and precision in introducing methyl groups to compounds, making it advantageous in pharmaceuticals, agrochemicals, and other chemical industries. This method has particularly impacted drug development, enabling the creation of more effective pharmaceuticals with fewer side effects, and it has improved agrochemical products by making them safer and more efficient.
Irvin's contributions extend beyond his scientific achievements; he has also been a mentor to many in the field, influencing the next generation of chemists. The Irvine-Purdie methylation continues to be a critical tool in chemistry and industry, underlining Irvin's lasting impact on the field.
In the realm of organic chemistry, Eric Jacobsen emerges as a distinguished figure, renowned for his pioneering research in asymmetric catalysis. His career, spanning several decades, is marked by significant contributions that have profoundly influenced modern chemistry, particularly through his work on Jacobsen epoxidation.
Born in the United States, Jacobsen's early fascination with chemistry propelled him towards pursuing an education in the field, culminating in a Ph.D. from Harvard University. His initial work laid the groundwork for a career characterized by innovation and discovery.
Upon completing his doctorate, Jacobsen joined the faculty of prestigious institutions, notably Harvard University, where he has been recognized with numerous awards and honors for his contributions to chemistry. These accolades reflect his work in catalysis and organic synthesis. His collaboration with Nobel Laureate Barry Sharpless stands out, merging Jacobsen's catalysis expertise with Sharpless's asymmetric synthesis knowledge to advance organic chemistry. This partnership underscores Jacobsen's potential for future Nobel recognition, highlighting the impact of collaborative innovation on scientific progress.
Jacobsen is best known for the Jacobsen epoxidation, a method for the enantioselective epoxidation of unfunctionalized olefins, utilizing chiral catalysts developed by his team. This breakthrough has significant implications, especially in pharmaceuticals, where enantiomerically pure compounds are crucial.
Beyond the Jacobsen epoxidation, his research encompasses a broad range of topics within asymmetric catalysis, including the development of novel catalytic systems and new synthetic pathways. Jacobsen's approach to research is defined by a deep understanding of chemical mechanisms, innovative problem-solving, and a dedication to advancing organic chemistry.
The Nobel Prize in Chemistry honors individuals whose scientific achievements have bestowed the greatest benefit to humankind. Eric Jacobsen's contributions, particularly in asymmetric catalysis and the Jacobsen epoxidation, make him a strong contender for this prestigious award. His work has not only pushed the boundaries of scientific knowledge but also found practical applications in drug development and manufacturing, benefiting society at large.
Jacobsen's journey in chemistry is marked by a relentless pursuit of knowledge and groundbreaking discoveries. His contributions have left an enduring mark on the field, and the scientific community's ongoing recognition and expansion of his work suggest the growing plausibility of Jacobsen receiving a Nobel Prize, celebrating a career that has significantly advanced our understanding of chemistry.
Marc Julia was a French chemist, born on October 23, 1922, in Paris. He earned his doctorate at the Collège de France under the guidance of Paul Vieille and later became a professor at the prestigious Institut de Chimie des Substances Naturelles in Gif-sur-Yvette. Julia is best known for the reaction named after him, the Julia olefination, which enables the synthesis of alkenes from sulfone precursors. The reaction is notable for its high E/Z-selectivity and has found widespread use in pharmaceutical and natural product synthesis. It offers an elegant alternative to the Wittig reaction and allows precise control over the double bond geometry. At ChemCon, the Julia olefination is used to construct well-defined olefin structures in drug intermediates – a critical step in ensuring the target compound's biological activity.
Henry Gilman (1911–1986), an eminent American chemist, is celebrated as the father of organometallic chemistry. Born on December 1, 1911, in Boston, Massachusetts, Gilman's academic journey led him to a Ph.D. from Harvard University in 1935 under the guidance of E.C. Kendall.
His illustrious career included academic positions at institutions such as Iowa State University, the University of Iowa, and Iowa State College. Gilman's groundbreaking contributions to organometallic chemistry established him as a key figure in the field.
One of his most notable achievements was the development of the Gilman reagent—a lithium or copper organocuprate compound widely utilized in organic synthesis for forming carbon-carbon bonds. His research delved into the synthesis, structure, and reactivity of compounds containing metal-carbon bonds, contributing significantly to the understanding of metalation reactions, where a metal replaces a hydrogen atom in an organic molecule.
Henry Gilman's legacy extends beyond his innovative methodologies. He received prestigious accolades for his work, including the Priestley Medal, the highest honor bestowed by the American Chemical Society (ACS). He was elected to the National Academy of Sciences and recognized as a fellow of the American Academy of Arts and Sciences. Gilman also held membership in the Royal Society of Chemistry.
The enduring impact of Gilman's contributions is reflected in the continued use of the Gilman reagent and related reactions in organic synthesis. His dedication to advancing organometallic chemistry has left an indelible mark, making him a revered figure in the scientific community and securing his place as a pioneer in the realm of chemistry.
Heinrich Emil Albert Knoevenagel...
..., born in Hannover, was a German chemist.
After studies in Hannover and Göttigen he got his PhD In 1889. Knoevenagel followed Victor Meyer to Heidelberg and became his assistant there. He habilitated in Heidelberg in 1892 with the topic of "asymmetric carbon". Emil Knoevenagel works at the University of Heidelberg as a Professor and works on nitrogen-heterocycles compounds. The preparation of unsaturated carbonyl compounds is named after him as the Knoevenagel reaction. A special example of Aldol condensation.
Ludwig Knorr's journey into the annals of organic chemistry is a narrative of intellectual pursuit, mentorship, and groundbreaking discoveries that have left a lasting imprint on the field. Born in Munich, Bavaria, on December 2, 1859, Knorr's early academic inclinations led him towards the study of medicine. However, it was chemistry that captured his heart and redirected his path towards becoming one of the most influential organic chemists of his time.
Knorr's academic odyssey took a definitive turn when he enrolled at the University of Erlangen. It was here that he came under the tutelage of Emil Fischer, a name synonymous with organic chemistry and its profound developments during the late 19th and early 20th centuries. Fischer, who would later be awarded the Nobel Prize in Chemistry in 1902 for his work on sugar and purine syntheses, served as Knorr's doctoral advisor. This mentorship was pivotal, placing Knorr at the forefront of organic chemistry research.
Under Fischer's guidance, Knorr embarked on his doctoral research, which culminated in the development of the Knorr synthesis of pyrrole, a fundamental building block in organic synthesis. His work laid the groundwork for synthesizing pyrrole derivatives, crucial in the manufacture of pharmaceuticals, dyes, and agrochemicals. This achievement was a testament to the influence Fischer had on Knorr, instilling in him a rigorous approach to research and an innovative spirit that would characterize his entire career.
Perhaps the most celebrated of Knorr's contributions is the development of the Knorr quinoline synthesis. This elegant chemical reaction involves the condensation of aniline with β-keto esters to form quinoline or its derivatives, compounds of significant importance in the pharmaceutical industry due to their therapeutic properties. The Knorr quinoline synthesis not only highlighted his genius in organic synthesis but also underscored the impact of his work on practical applications, particularly in medicine.
Throughout his career, Ludwig Knorr was honored with numerous accolades, reflecting his pivotal role in advancing organic chemistry. Yet, it was his collaboration and intellectual kinship with Emil Fischer that perhaps most profoundly shaped his approach to science. Fischer's mentorship provided Knorr with a solid foundation in the principles of organic chemistry, which he built upon to achieve his remarkable scientific contributions.
Knorr's legacy extends beyond his chemical syntheses; it lies in his ability to merge theoretical chemistry with practical applications, influencing subsequent generations of chemists. His work continues to be a cornerstone in the fields of pharmaceuticals and organic synthesis, embodying the enduring impact of a scientist whose pursuit of knowledge was matched by his contributions to humanity's well-being.
Wilhelm Koenigs was a German chemist active in the late 19th century. Together with fellow German chemist Eduard Knorr, he developed a method for glycoside synthesis now known as the Koenigs–Knorr method. This reaction uses activated sugar halides and alcohols to selectively form glycosidic bonds – a key step in building complex carbohydrate structures. The method was a milestone in carbohydrate chemistry and laid the foundation for many modern approaches to synthesizing bioactive sugar derivatives. At ChemCon, the Koenigs–Knorr method is used in the synthesis of functionalized glycosides that serve as pharmaceutical excipients or active intermediates.
Hermann Kolbe was born in Elliehausen in 1818. In 1838 he passed his Abitur in Göttingen and began his chemistry studies at the Georg-August University. From 1842 he became an assistant to Robert Wilhelm Bunsen in Marburg. He completed his doctorate in autumn 1843 with the thesis "Ueber die Produkte der Einwirkung des Chlors auf Schwefelkohlenstoff". From 1845 to 1847 Kolbe was assistant to Lyon Playfair at the University of London. During his time in London, he discovered with Edward Frankland the mode of preparation of carboxylic acids from nitriles. At the same time, Kolbe also found a way to prepare dimerised alkanes using electrolysis of carboxylic acids. In 1851 he became Bunsen's successor at the University of Marburg. He became a full professor at the University of Leipzig in 1865. Here the "Chemical Institute of the University of Leipzig" was built according to his plans in 1868.
The Kolbe-Schmitt reaction was particularly significant for medicine. Salicylic acid, especially its derivative acetylsalicylic acid (ASA), is an active ingredient that relieves fever and pain, among other things.
ASA has been on the WHO's list of essential medicines since 1977. The active ingredient has been marketed in various products since the beginning of the 20th century.
Nathan Kornblum, a prominent 20th-century chemist, was born in 1914 and left a lasting impact on organic chemistry. His scientific journey began with a degree in chemistry from Columbia University, followed by a Ph.D. from the University of Wisconsin-Madison. He then embarked on an academic career that ultimately led him to Purdue University, where he became a respected professor and researcher known for his meticulous and groundbreaking work.
One of his most significant contributions to chemistry is the Kornblum oxidation, which he developed in the 1950s. This reaction has had a profound influence on organic synthesis and is still widely used in research and industry today. The Kornblum oxidation is a method for converting primary alkyl halides into aldehydes, while secondary alkyl halides are oxidized to ketones. The reaction uses DMSO as the oxidizing agent, and often a base such as triethylamine is added to neutralize the resulting hydrogen halide derivative. This reaction is particularly valuable because it can be carried out under relatively mild conditions, providing an alternative to harsher oxidizing agents.
However, Kornblum's academic achievements were not limited to this reaction alone. Throughout his career, he published numerous scientific papers and mentored many Ph.D. students, some of whom went on to become prominent scientists in their own right. His work significantly advanced the development of new organic synthesis methods, which have found broad application in the pharmaceutical industry and material science.
Despite his impressive academic accomplishments, Kornblum was known for his humility and passion for helping young scientists. He was always eager to share his knowledge in an accessible way, and he was highly respected by both his students and colleagues.
Nathan Kornblum passed away in 1978, but his scientific legacy lives on. His contributions, particularly the Kornblum oxidation, remain fundamental methods in organic chemistry and highlight his lasting place in the history of science.
Oleg Grigorjewitsch Kulinkowitsch, a distinguished Belarusian chemist, made significant contributions to organic chemistry, particularly in the field of synthetic methodologies. Born in the Soviet Union, Kulinkowitsch developed a passion for chemistry early in his life. His academic journey began with a strong foundation in chemical sciences, leading him to pursue advanced studies and research, where his work would eventually earn him recognition on an international scale.
Kulinkowitsch's career was marked by a deep commitment to exploring new chemical reactions and understanding their applications. His innovative approach to organic synthesis led to the discovery of what is now known as the Kulinkovich reaction, a groundbreaking method in the synthesis of cyclopropanol derivatives. This reaction involves the transformation of carboxylic acid esters into cyclopropanols, using a Grignard reagent in the presence of a titanium(IV) alkoxide as a catalyst. The simplicity and efficiency of this reaction have made it an invaluable tool in the toolkit of organic chemists.
The Kulinkovich reaction is particularly noteworthy not only for its elegance but also for its broad applicability in synthetic organic chemistry. One of the key areas where this reaction has found significant use is in the synthesis of complex molecules, including those with biological importance such as hormones. Cyclopropanols, the products of the Kulinkovich reaction, are versatile intermediates that can be further manipulated to create various biologically active compounds. This has opened up new avenues in medicinal chemistry, where the synthesis of hormone analogs and other pharmaceuticals often requires the construction of challenging molecular frameworks.
The utility of the Kulinkovich reaction in hormone synthesis is particularly evident in the production of steroids and other hormone-like compounds. The ability to efficiently construct cyclopropane rings, which are common structural motifs in many natural products and pharmaceuticals, has provided chemists with a powerful method to develop new drugs and study hormone functions. This has not only advanced the field of organic chemistry but also contributed to the development of new treatments and therapeutic agents.
Throughout his career, Oleg Grigorjewitsch Kulinkowitsch remained dedicated to pushing the boundaries of chemical research. His work has inspired countless chemists to explore new reactions and develop innovative solutions to complex synthetic challenges. The legacy of his eponymous reaction continues to influence the field, demonstrating the lasting impact of his contributions to science.
In addition to his research achievements, Kulinkowitsch was known for his mentorship and collaboration with other scientists. He was deeply committed to fostering the next generation of chemists, sharing his knowledge and passion for discovery. His influence extended beyond his own laboratory, as his methods and ideas were adopted and expanded upon by researchers worldwide.
Oleg Grigorjewitsch Kulinkowitsch's life and work exemplify the profound impact that a single scientist can have on their field. His contributions to organic chemistry, particularly through the development of the Kulinkovich reaction, have left an indelible mark on the scientific community. His methods continue to enable advances in the synthesis of important molecules, including hormones, underscoring the importance of innovation and creativity in scientific research.
Raymond U. Lemieux (1920–2000) was a pioneering Canadian organic chemist whose groundbreaking work in carbohydrate chemistry transformed the field and paved the way for advancements in medicine, biochemistry, and pharmaceuticals. His contributions, particularly in glycoside synthesis and carbohydrate structure elucidation, laid the foundation for modern glycoscience.
Born in Lac La Biche, Alberta, Lemieux developed an early interest in chemistry, which led him to study at the University of Alberta. He completed his Ph.D. at McGill University under the supervision of Carl Niemann, focusing on carbohydrate chemistry—a field that, at the time, was still in its infancy.
One of Lemieux’s most significant contributions was his work on glycoside synthesis. In 1953, he and his team successfully synthesized sucrose, marking the first time a complex sugar was artificially created. This achievement not only deepened the understanding of sugar structures but also had major implications for pharmaceuticals and biotechnology.
Lemieux’s research on glycosylation—the process of attaching sugar molecules to other biomolecules—led to innovations in antibiotics, blood group antigens, and vaccine development. His work directly influenced the production of heparin and other carbohydrate-based drugs, which are essential in anticoagulation therapy and immune response modulation.
Another notable contribution was the development of the Lemieux-Johnson oxidation reaction, a method for oxidative cleavage of alkenes into carbonyl compounds using osmium tetroxide and periodate. This reaction remains widely used in organic synthesis and pharmaceutical research, significantly impacting drug design and development. His pioneering work has influenced modern pharmaceutical science, particularly in the synthesis of complex carbohydrates used in vaccines, therapeutics, and diagnostic tools.
Beyond academia, Lemieux played a crucial role in the pharmaceutical industry. He founded the biotech company Chembiomed, which focused on carbohydrate-based drug development. Additionally, his contributions to understanding the role of carbohydrates in biological systems have had lasting impacts on immunology and disease research.
Lemieux received numerous accolades for his contributions to chemistry, including the prestigious Wolf Prize in Chemistry and the Royal Medal from the Royal Society of London. His work not only established Canada as a leader in carbohydrate chemistry but also set the stage for future discoveries in glycobiology and medicinal chemistry.
Raymond Lemieux’s legacy continues to influence scientific advancements today, underscoring the importance of carbohydrates in life sciences and therapeutic development. His pioneering research remains fundamental to modern chemistry, proving that even the most complex molecules can be understood and harnessed for the benefit of humanity.
Wilhelm Clemens Lossen was born on May 8, 1838, in Kreuznach, now known as Bad Kreuznach, Germany. His educational journey in chemistry included significant mentorships; notably, he served as an assistant to Karl Weltzien in Karlsruhe and to Wilhelm Heinrich Heintz in Halle. His academic career progressed as he took on the role of an associate professor at the University of Heidelberg and later became a full professor of chemistry at the University of Königsberg.
Lossen is perhaps best known for the Lossen rearrangement, an organic chemical reaction involving the transformation of hydroxamic acids into isocyanates using a dehydrating agent. This reaction has been widely recognized for its importance in the synthesis of various organic compounds, particularly in the pharmaceutical industry, where it plays a crucial role in the development of new drugs.
Throughout his career, Lossen was deeply engaged with the academic and scientific communities, contributing extensively through both teaching and research. His approach to education and mentorship left a lasting impact on his students and colleagues, fostering a legacy of inquiry and discovery.
Wilhelm Clemens Lossen passed away on October 29, 1906, in Aachen. His contributions to the field of chemistry continue to be celebrated and remain integral to various scientific applications today.
Carl Ulrich Franz Mannich was born in Breslau in 1877. He began his pharmacy studies in Marburg and Berlin in 1898. In 1903, he earned his doctorate in Basel and became a habilitated lecturer in Berlin in 1907. From 1911 to 1917, he taught pharmaceutical chemistry in Göttingen. In 1920, he moved to Frankfurt am Main. Afterward, Mannich served as a professor of pharmaceutical chemistry at the Friedrich-Wilhelms-Universität Berlin and simultaneously as the director of the pharmaceutical institute until 1943. In 1946, he took over the chair of pharmaceutical chemistry at what is now the Karlsruhe Institute of Technology.
From 1932 to 1934, he was the president of the "Deutsche Pharmazeutische Gesellschaft" (German Pharmaceutical Society), which has been awarding the Carl Mannich Medal for "outstanding achievements in the field of scientific pharmacy" since 1959.
The Mannich Reaction is applied in the synthesis of natural substances, the production of pharmaceuticals, and plant protection agents, as well as in the fields of paint and polymer chemistry.
At ChemCon, as a Contract Development and Manufacturing Organization (CDMO), we have over 25 years of experience in the development and manufacturing of Active Pharmaceutical Ingredients (API) under the highest Good Manufacturing Practice (GMP) conditions. Our portfolio includes active ingredients used in cancer therapy, ophthalmology, and for treating rare diseases (orphan diseases).
In addition to contract development, ChemCon has established a renowned laboratory for contract analytics, operating in accordance with GMP and ICH guidelines, as well as pharmacopeia standards such as the European Pharmacopoeia.
For several years now, we synthesize polymers, particularly Polyethylenimines (PEI) and Polyoxazolines (PxOx), in our labs. These substances can be used in the pharmaceutical industry as reagents for nucleic acid separation, and PEIs are also used as transfection agents. To be used as excipients in medications, they must be manufactured according to GMP, an area in which ChemCon has extensive experience.
John E. McMurry is a distinguished American chemist known for his significant contributions to organic chemistry. Born in 1942, McMurry pursued his academic journey with a deep focus on synthetic methodologies. After earning his Ph.D. from Columbia University under the guidance of Gilbert Stork, he embarked on a successful career in both research and education. As a professor at Cornell University, McMurry became widely recognized not only for his groundbreaking research but also for his influential textbooks, which continue to shape how organic chemistry is taught worldwide.
One of McMurry’s most notable scientific achievements is the development of the McMurry reaction, a powerful method for forming carbon-carbon double bonds. This reaction involves the reductive coupling of two carbonyl compounds—either aldehydes or ketones—using low-valent titanium reagents. The resulting alkenes are valuable intermediates in organic synthesis, providing a versatile approach to constructing complex molecules. The McMurry reaction has become a cornerstone technique in modern organic chemistry due to its efficiency and ability to produce otherwise challenging molecular structures.
Beyond its academic importance, the McMurry reaction plays a crucial role in the pharmaceutical industry. It enables the synthesis of structurally complex compounds, including biologically active molecules, which are essential in drug development. This methodology is particularly useful for creating unique chemical scaffolds and exploring new therapeutic candidates. By facilitating the construction of intricate carbon frameworks, the reaction has opened pathways to produce novel pharmaceuticals and advanced materials.
At Chemcon, the McMurry reaction is one of many advanced techniques employed in custom synthesis projects. The ability to manipulate carbonyl compounds with precision aligns with Chemcon’s expertise in delivering specialized chemical solutions. Whether supporting early-stage research or full-scale production, the use of such methodologies allows Chemcon to provide tailored compounds that meet the highest standards of quality and regulatory compliance. This flexibility is particularly valuable in developing active pharmaceutical ingredients (APIs) and other critical materials where customized chemical synthesis is essential. By leveraging reactions like McMurry’s, Chemcon continues to support innovation and efficiency in the pharmaceutical and chemical sectors, offering clients the ability to transform complex chemical ideas into tangible products.
Arthur Michael...
... was an American chemist who never actually graduated the university. He acquired his knowledge of chemistry through to local teachers in his private laboratory, as he was unable to study at Harvard due to illness. He acquired further knowledge by visiting well-known chemists when he traveled to Europe. At Tufts College, he met his wife and worked as a professor of chemistry. In 1912, he went to Harvard University, where he served as a professor without lecture duties until 1936, despite the fact that he never earned a university degree. Nowadays, Arthur Michael is mainly known for the Michael Addition, which is named after him.
Oyo Mitsunobu, a distinguished Japanese chemist, is renowned for his significant contributions to organic chemistry, particularly through the reaction that bears his name the Mitsunobu reaction. Born in 1934 in Japan, Mitsunobu’s early life was marked by a keen interest in science and an unwavering dedication to his studies. He pursued his higher education at the University of Tokyo, where he eventually earned his Ph.D. in organic chemistry. His academic career was characterized by an intense focus on developing innovative chemical reactions, which would later cement his legacy in the scientific community.
Mitsunobu’s most notable achievement is the development of the Mitsunobu reaction, a transformative process in organic synthesis that allows the conversion of alcohols into a variety of other functional groups, including esters, ethers, amines, and thioethers. This reaction employs specific reagents such as diethyl azodicarboxylate (DEAD), diisopropyl azodicarboxylate (DIAD), or tert-butyl azodicarboxylate (TMAD), which facilitate the substitution of the hydroxyl group with nucleophiles. One of the hallmark features of the Mitsunobu reaction is its ability to induce an inversion of configuration at stereogenic centers. This means that secondary alcohols, when subjected to the reaction conditions, undergo a stereochemical inversion, making this reaction particularly valuable for the synthesis of enantiomerically pure compounds.
However, the Mitsunobu reaction is not without its drawbacks. One significant criticism is its poor atom economy. The reaction generates a substantial amount of by-products, which can complicate purification and reduce overall efficiency. Despite this limitation, the reaction remains highly valued for its versatility and ability to form carbon-nitrogen and carbon-oxygen bonds, especially in the context of converting hydroxyl groups into amine groups. This transformation is particularly useful in pharmaceutical synthesis, where precise modifications of molecular structures are often required.
Oyo Mitsunobu's influence on current science is profound. The Mitsunobu reaction has been the basis for numerous patents and scientific publications, reflecting its widespread application in both academic research and industrial processes. The reaction's utility in creating complex molecules has made it a staple in the toolkit of synthetic chemists worldwide. Its application extends beyond simple transformations, influencing the development of new methodologies and contributing to the synthesis of complex natural products and pharmaceuticals.
Throughout his career, Mitsunobu received several accolades for his work, underscoring his impact on the field of organic chemistry. His innovative approach and the subsequent development of the Mitsunobu reaction have inspired countless chemists to explore new frontiers in chemical synthesis. The reaction's enduring relevance in modern chemistry is a testament to Mitsunobu’s ingenuity and his lasting legacy in the scientific community.
Ei-ichi Negishi was one of the most prominent figures in the field of chemistry, best known for developing the Negishi coupling, a method used widely in the synthesis of complex organic compounds. Born in Changchun, China, in 1935, Negishi moved to Japan with his family at the end of World War II. He pursued his education in Japan, earning his bachelor's degree from the University of Tokyo in 1958.
Negishi's journey to global recognition began when he traveled to the United States to further his studies. He obtained his PhD in chemistry from the University of Pennsylvania in 1963 under the tutelage of famed chemist Allan R. Day. He later joined the group of another celebrated chemist, Herbert C. Brown, at Purdue University as a postdoctoral fellow. This association was pivotal, as Brown's work on organoboranes would earn him a Nobel Prize and deeply influenced Negishi's own research trajectory.
Negishi spent the majority of his career at Purdue University, where he started as an assistant professor in 1972 and was promoted to full professor in 1979. His research focused on developing methods for easily and efficiently linking carbon atoms together, a fundamental aspect of organic synthesis. The most significant breakthrough in Negishi's career came with the development of the Negishi coupling in the late 1970s. This powerful chemical reaction involves the cross-coupling of organozinc compounds with aryl or alkenyl halides, facilitated by a palladium or nickel catalyst. The elegance and efficiency of this reaction made it a valuable tool for constructing complex, multi-functional organic compounds, including pharmaceuticals, natural products, and advanced materials.
The importance of Negishi's work was recognized in 2010 when he was awarded the Nobel Prize in Chemistry, along with Richard F. Heck and Akira Suzuki. The trio was honored for their contributions to the development of palladium-catalyzed cross couplings in organic synthesis, which have revolutionized the way chemists build complex molecules. The Nobel Committee specifically highlighted the broad applicability of these methods in both academic and industrial settings, noting their role in making sophisticated chemical manufacturing more practical and environmentally friendly.
Throughout his career, Negishi was not only a brilliant chemist but also a dedicated mentor and educator. He was passionate about inspiring the next generation of scientists, often emphasizing the importance of curiosity, perseverance, and fundamental understanding in scientific inquiry. His teachings and philosophies have left a lasting impact on many of his students and colleagues.
Ei-ichi Negishi's contributions to chemistry are profound, extending beyond the laboratory to affect various industries, including pharmaceuticals and materials science. His work exemplifies the power of chemical innovation to solve complex problems and improve lives. The legacy of his scientific achievements and his commitment to education continue to inspire and influence the field of chemistry today.
Ronald George Wreyford Norrish, born on November 9, 1897, in Cambridge, England, was destined for a life steeped in academic excellence. His father, George Norrish, was a pharmacist, a profession demanding precision and analytical thinking—traits that young Ronald would carry into his scientific pursuits. The intellectual environment of Cambridge undoubtedly played a crucial role in shaping Norrish's future, providing him with access to some of the best educational resources in the world.
Norrish's academic journey began at the prestigious Perse School in Cambridge, followed by his studies at Emmanuel College, University of Cambridge. His education was briefly interrupted by his service in World War I, where he was wounded in action. After the war, he returned to Cambridge, completing his degree in chemistry in 1921.
Norrish's brilliance in the field of chemistry quickly became evident. He was appointed a lecturer at Cambridge in 1925 and eventually became a professor of physical chemistry. His work was instrumental in advancing the understanding of chemical kinetics and photochemistry. Norrish's research was characterized by a deep curiosity about the mechanisms of chemical reactions and how light could influence these processes.
Norrish's most significant contributions lie in the field of photochemistry, the study of chemical reactions that occur due to the absorption of light. He explored the photochemical decomposition of carbonyl compounds, a class of organic compounds containing a carbon-oxygen double bond. His work in this area laid the foundation for much of modern photochemistry.
One of Norrish's key discoveries was the Norrish reaction, which describes the photochemical cleavage of carbonyl compounds. Upon absorbing a photon, the carbonyl group is excited to a singlet state. This excited state can either proceed to form a triplet state or directly undergo bond cleavage to produce free radicals. These free radicals then participate in various subsequent reactions, forming the basis for numerous photochemical processes in organic chemistry.
Another important phenomenon associated with Norrish is the Trommsdorff-Norrish effect, which refers to the autoacceleration of polymerization reactions. When certain polymers are exposed to light, the rate of polymerization increases rapidly due to the formation of reactive species. However, this effect is not limited to light-induced polymerizations; it can also occur in thermally initiated polymerizations and other types of chain reactions. This effect is particularly significant in the production of synthetic polymers and has industrial applications in creating materials with specific properties.
Ronald Norrish's groundbreaking work did not go unrecognized. In 1967, he was awarded the Nobel Prize in Chemistry, sharing the honor with his colleague George Porter and the German chemist Manfred Eigen. The Nobel Committee recognized their combined efforts in studying extremely fast chemical reactions, measured in nanoseconds. Norrish and Porter's development of the flash photolysis technique was particularly noteworthy. This method allowed chemists to observe the intermediate stages of photochemical reactions, revolutionizing the understanding of reaction mechanisms.
Ronald George Wreyford Norrish passed away on June 7, 1978, leaving behind a legacy of scientific innovation and discovery. His work continues to influence chemists today, particularly in the fields of physical chemistry and photochemistry. The Norrish reaction and the Trommsdorff-Norrish effect remain integral to understanding chemical processes, both in academic research and industrial applications. Norrish's life is a testament to the power of curiosity and perseverance in scientific inquiry. His contributions have paved the way for future generations of chemists to explore the intricate dance of molecules under the influence of light, pushing the boundaries of what we know about the chemical world.
Tamejiro Hiyama is a distinguished Japanese chemist whose groundbreaking contributions to the field of organic and organometallic chemistry have had a profound impact on modern synthetic methodologies, particularly in pharmaceutical chemistry. His work has not only advanced our understanding of catalytic processes but also provided innovative tools for constructing complex molecular architectures.
Born in Japan in 1947, Tamejiro Hiyama showed an early interest in science and chemistry. He pursued his undergraduate studies at Kyoto University, one of Japan's most prestigious institutions. Hiyama continued at Kyoto University for his doctoral studies, earning a Ph.D. in 1974 under the mentorship of notable chemists who inspired his early foray into organometallic chemistry.
After completing his doctorate, Hiyama gained valuable international experience as a postdoctoral researcher at Purdue University in the United States, where he honed his expertise in synthetic chemistry and catalysis. This exposure to diverse scientific environments shaped his future research direction.
Hiyama returned to Japan, where he embarked on an illustrious academic career. He held faculty positions at Kyoto University and later at Nagoya University, two leading centers for chemical research. Over the decades, he established himself as a leading figure in organometallic chemistry, mentoring numerous students and fostering international collaborations.
Hiyama's scientific contributions are both diverse and impactful. Among his most significant achievements is the development of the Nozaki-Hiyama-Kishi (NHK) reaction, a method for carbon-carbon bond formation. The reaction, co-developed with Hideo Nozaki and Kishi Yoshito, involves the coupling of alkyl or allyl halides with aldehydes in the presence of chromium or nickel catalysts. This methodology has become a cornerstone in synthetic organic chemistry, allowing for the efficient construction of secondary and tertiary alcohols.
The NHK reaction is particularly valued for its ability to create complex carbon skeletons, which are often found in natural products and pharmaceutical compounds. Its versatility and efficiency have made it a staple in the toolkit of synthetic chemists worldwide.
Hiyama's contributions extend beyond fundamental chemistry to real-world applications in drug discovery and development. The NHK reaction, for instance, has been instrumental in the synthesis of biologically active molecules, including natural products with therapeutic potential. By enabling precise and efficient bond formation, Hiyama's methodologies have streamlined the synthesis of complex pharmaceutical compounds, reducing costs and improving scalability.
Additionally, Hiyama has worked extensively on silicon-based reagents, developing protocols that have enhanced the selectivity and efficiency of organic transformations. These innovations have further cemented his influence in medicinal chemistry, where precision and adaptability are paramount.
Tamejiro Hiyama's contributions to chemistry have earned him widespread recognition. He has received numerous awards, including the Chemical Society of Japan Award and the Humboldt Research Award, underscoring his international impact. Beyond his accolades, his legacy endures through the countless chemists he has mentored and the enduring relevance of his methodologies.
Today, Tamejiro Hiyama remains an inspiring figure in the global chemical community. His work exemplifies the power of fundamental research to drive innovation in practical applications, particularly in areas as vital as pharmaceutical chemistry. As new generations of chemists build on his foundations, Hiyama's influence will undoubtedly continue to shape the future of science and medicine.
Larry E. Overman, a distinguished American chemist, is renowned for his contributions to the field of organic chemistry, particularly for the Overman rearrangement. His pioneering work has profoundly influenced synthetic chemistry, especially in the synthesis of complex natural products and pharmaceutical compounds.
Born in 1943 in Chicago, Illinois, Overman displayed an early interest in science, which paved the way for his future academic pursuits. He received his Bachelor of Science degree in Chemistry from Earlham College, a liberal arts college known for its emphasis on undergraduate research. Following this, Overman pursued his PhD at the University of Wisconsin-Madison, where he worked under the tutelage of Howard E. Zimmerman. His doctoral research focused on photochemical reactions, a theme that would recur throughout his career.
After completing his PhD, Overman accepted a position at the University of California, Irvine, where he has spent the majority of his academic career. Here, he made significant advancements in organic synthesis, developing new methods that have become essential tools for chemists around the world. Among his many achievements, the Overman rearrangement stands out. This reaction, developed in the 1970s, is a method for converting allylic alcohols into allylic amines using trichloroacetimidate as a key reagent. The Overman rearrangement is particularly notable for its ability to efficiently create chiral amine structures, which are pivotal in the synthesis of many biologically active molecules.
The impact of the Overman rearrangement extends beyond theoretical chemistry. It has practical applications in the synthesis of complex molecules, particularly in the pharmaceutical industry. For instance, it has been used in the development of polyallylamine compounds, which are crucial for creating high-performance polymers. These polymers have various industrial applications, including water treatment and enhancement of paper strength.
Moreover, the Overman rearrangement has played a critical role in the synthesis of Sevelamer, a phosphate binding drug used to treat hyperphosphatemia in patients with chronic kidney disease. The synthesis of Sevelamer showcases the practical health implications of Overman's work, illustrating how fundamental chemical reactions can lead to significant advancements in medicine.
Throughout his career, Overman has been recognized with numerous awards and honors, reflecting his status as a leader in the field of chemistry. His influence is also evident in his role as a mentor to young chemists, many of whom have gone on to make their own significant contributions to science.
In conclusion, Larry E. Overman's work exemplifies how deep theoretical knowledge combined with a commitment to practical application can lead to advancements that extend far beyond the laboratory. His development of the Overman rearrangement has not only expanded the toolkit of organic chemists but has also facilitated significant progress in the development of pharmaceuticals and industrial materials. His legacy is a testament to the power of chemistry to solve complex problems and improve human lives.
Emanuele Paternò was a pivotal figure in the field of chemistry whose contributions still resonate within the scientific and pharmaceutical communities today. Born in Palermo, Italy, in 1847, Paternò grew up in a period of burgeoning scientific curiosity and development. Early on, his natural affinity for the sciences set him on a path toward a career that would leave a lasting impact. He studied under the guidance of influential chemists of his time and developed an inquisitive, rigorous approach that he carried throughout his career. After completing his studies, Paternò’s academic path took him across Europe, where he absorbed and contributed to the growing body of chemical knowledge, which ultimately culminated in his notable discovery of the Paternò-Büchi reaction.
This photochemical reaction, known as the Paternò–Büchi reaction, stands as a hallmark of his work and remains a fundamental tool in organic synthesis today. In this reaction, carbonyl compounds interact with alkenes under the influence of light, forming a four-membered oxetane ring. This discovery was groundbreaking because it showcased the potential of photochemistry in creating complex molecular structures—a notion that was still largely unexplored at the time. By harnessing the energy from light to drive chemical transformations, Paternò demonstrated how reactions that seemed implausible under traditional conditions could be realized. The [2+2] cycloaddition mechanism he pioneered not only opened new synthetic pathways but also laid the groundwork for advancements in organic photochemistry, inspiring further research into how light can be leveraged to influence chemical reactions.
Paternò’s work reached beyond academia, having profound implications for modern chemistry and, by extension, the pharmaceutical industry. The ability to create oxetane rings through photochemical reactions introduced novel possibilities in drug design and development, as these four-membered rings possess unique chemical properties that can enhance the activity and stability of pharmaceutical compounds. Today, oxetane-containing molecules are actively explored in drug research for their potential to modify biological targets with high specificity, enhancing the effectiveness of treatments in fields ranging from oncology to antimicrobial therapy. This approach, rooted in Paternò’s initial discovery, illustrates the far-reaching implications of his research and its role in shaping modern medicinal chemistry.
Throughout his career, Paternò was also deeply involved in education and scientific communication. He mentored a generation of chemists who went on to further advance the field, spreading his principles of rigorous experimentation and curiosity-driven research. His legacy is not just limited to his own discoveries but is reflected in the work of countless others who built upon his ideas and techniques. By bridging the gap between photochemistry and organic synthesis, Emanuele Paternò left an indelible mark on the scientific landscape, ensuring that his contributions would benefit future generations and the ongoing progress of chemistry and pharmaceuticals. His work endures as a testament to the power of innovation, curiosity, and the transformative impact of fundamental research.
Mario Passerini was a pioneering Italian chemist whose contributions to organic chemistry continue to shape modern scientific advancements. His most notable achievement, the Passerini reaction, remains a cornerstone in the synthesis of complex molecules. Born in the early 20th century, Passerini dedicated his academic career to studying multicomponent reactions, leading to the discovery of his eponymous reaction. This transformation, which allows the efficient formation of α-hydroxy carboxamide esters from carbonyl compounds, isocyanides, and carboxylic acids, revolutionized organic synthesis by enabling the construction of structurally diverse compounds in a single step.
The Passerini reaction has had a profound impact on the pharmaceutical industry, providing an efficient pathway for the development of biologically active molecules. Its ability to generate molecular complexity with high atom economy has made it a valuable tool in drug discovery and medicinal chemistry. Many modern pharmaceuticals leverage multicomponent reactions inspired by Passerini’s work, facilitating the rapid synthesis of compound libraries for screening new therapeutic agents. His research laid the groundwork for advances in both natural product synthesis and polymer chemistry, influencing the development of functionalized materials used in medical applications.
ChemCon is a forward-looking player in the field of medicinal chemistry and GMP-polymers, building upon the foundations established by pioneers like Passerini. The company specializes in custom synthesis, developing high-purity compounds that meet stringent regulatory requirements. As pharmaceutical research continues to evolve, the ability to produce specialized molecules with precision and consistency is crucial. ChemCon’s expertise in GMP-polymers ensures that innovative materials are available for advanced medical applications, from drug delivery systems to bio-compatible coatings. With a strong focus on quality and innovation, ChemCon bridges the gap between groundbreaking research and real-world applications, demonstrating the lasting relevance of Passerini’s work in today’s pharmaceutical and material sciences industries.
Peter L. Pauson, a prominent figure in the world of chemistry, made significant contributions to the field of organometallic chemistry. Born on August 23, 1925, in Berlin, Germany, Pauson grew up during a time of considerable global changes, eventually relocating to the United Kingdom. He pursued his academic studies at University College London, where he developed a strong foundation in chemistry that would later define his career.
Pauson’s most notable achievement is the development of the Pauson-Khand reaction, a collaboration with Ihsan Khand. This reaction has become a cornerstone in synthetic organic chemistry due to its efficiency and versatility. The Pauson-Khand reaction is a 2+2+1 cycloaddition process that involves the interaction of an alkene, an alkyne, and carbon monoxide, facilitated by the presence of dicobalt octacarbonyl as a catalyst. This reaction results in the formation of substituted cyclopentenones, which are essential in synthesizing complex organic molecules. The ability to construct five-membered rings, prevalent in natural products and pharmaceuticals, has made this reaction an invaluable tool for chemists.
The impact of Pauson’s work extends far beyond the Pauson-Khand reaction. His pioneering efforts in organometallic chemistry have paved the way for further innovations in synthesizing complex molecules and studying metal-organic frameworks. His research has influenced the development of new catalytic processes and advanced the understanding of chemical reactions and mechanisms.
Peter Pauson’s legacy in chemistry is marked by his dedication to the field and his contributions to advancing modern chemistry. His work continues to inspire and influence chemists worldwide, reflecting the profound impact he has had on the scientific community.
Hans von Pechmann, born on April 1, 1850, in Mülhausen, Alsace, was a pioneering figure in the field of organic chemistry. His journey in science began with his studies at the University of Munich under the mentorship of Adolf von Baeyer, where he developed a profound interest in the synthesis of organic compounds.
Pechmann's contributions to chemistry are numerous, with his development of the Pechmann condensation reaction being among the most notable. This reaction, which synthesizes coumarins from phenols and β-keto esters in the presence of acids, has opened new pathways in the synthesis of natural products and heterocyclic compounds, crucial for pharmaceuticals and dyes.
Another significant achievement of Pechmann was the discovery of Polyethylene in 1898, a result of his experiments with diazomethane. This discovery laid the foundation for the development of plastics, revolutionizing industries from packaging to telecommunications. Nowadays, polymers are extensively used for pharmaceutical applications, including the production of medicines where Good Manufacturing Practice (GMP) grade polymers serve as excipients, enhancing the stability, efficacy, and delivery of active pharmaceutical ingredients. Their durability and flexibility have made polymers indispensable in the modern world.
Pechmann's work did not just contribute to the advancement of chemical synthesis and polymer science; it also inspired future generations of chemists. Despite passing away on April 19, 1902, his legacy lives on through the Pechmann reaction and the widespread use of polymers, underscoring the impact of his innovations on modern life and the field of chemistry.
Sir William Henry Perkin, born on March 12, 1838, in London, was a renowned chemist whose accidental discovery revolutionized the textile industry and left an indelible mark on the world of science.
Perkin's journey into chemistry began at an early age when he showed a keen interest in science. He pursued his academic endeavors at the Royal College of Chemistry under the mentorship of August Wilhelm von Hofmann, a distinguished chemist of his time.
In 1856, while attempting to synthesize quinine, a drug for treating malaria, Perkin stumbled upon an unexpected breakthrough. Instead of quinine, he discovered a vibrant purple dye, later named "mauveine" or "mauve." This accidental creation proved to be a game-changer in the textile industry, offering a cost-effective alternative to natural dyes and sparking a revolution in color.
Recognizing the commercial potential of his discovery, Perkin founded a chemical manufacturing company to produce synthetic dyes on an industrial scale. His entrepreneurial spirit and scientific prowess propelled him to great success, making him a leading figure in the chemical industry.
Perkin's contributions extended beyond the discovery of mauveine. He continued to innovate, introducing a range of synthetic dyes that transformed the fashion industry and found applications in printing and photography.
In addition to his achievements in industry, Perkin's academic pursuits were equally remarkable. His studies at the Royal College of Chemistry laid the groundwork for his groundbreaking research, and his mentorship under Hofmann nurtured his passion for chemistry.
Perkin's legacy endures as a testament to the power of scientific curiosity and innovation. His accidental discovery of mauveine not only revolutionized the textile industry but also reshaped the way we perceive color and creativity.
Knighted in 1906 for his contributions to chemistry, Sir William Henry Perkin remains a celebrated figure in the scientific community, his name synonymous with progress and ingenuity in the field of chemistry.
Amé Pictet, born in Geneva in 1857, was a distinguished Swiss chemist whose work significantly influenced both organic chemistry and pharmaceutical science. Educated at the University of Geneva, Pictet delved deeply into the field of heterocyclic chemistry, contributing to scientific knowledge in ways that would later underpin key developments in pharmacology. His early career was marked by a dedication to understanding complex organic molecules, which led to significant advancements and insights that have had a lasting impact.
One of his notable contributions is the Pictet-Gams synthesis, a chemical reaction he developed with Austrian chemist Theodor Gams. This synthesis forms part of a larger group of reactions bearing his name, including the Pictet-Spengler reaction. The Pictet-Gams reaction specifically focuses on the formation of isoquinoline derivatives, which are foundational in producing compounds with a variety of medicinal applications. Isoquinolines are nitrogen-containing heterocycles and serve as core structures in many natural and synthetic alkaloids, some of which are utilized in pain relief, antimalarial treatments, and even oncology. The capacity of isoquinolines to interact with diverse biological pathways has made them indispensable in drug design and development, enabling researchers to create more effective therapies by tailoring their interactions with specific cellular targets.
The Pictet-Gams synthesis facilitates the formation of these compounds with a level of efficiency and precision that was groundbreaking at the time. By combining an arylamine with an aldehyde or ketone, followed by acid-mediated cyclization, chemists can synthesize these complex structures in fewer steps, yielding purer results. This method not only advanced synthetic chemistry but also provided the pharmaceutical industry with a reliable pathway for producing drugs with isoquinoline backbones. Derivatives of isoquinoline have been used to develop a broad range of pharmaceuticals, from analgesics like morphine to antitussives and certain anticancer drugs.
Pictet’s achievements in chemistry garnered him recognition across academic and professional circles, earning several honorary positions and awards. He published extensively, with his research being celebrated for its methodological rigor and innovative nature. The impact of Pictet’s work on medicinal chemistry cannot be overstated; the tools and techniques he developed continue to shape how chemists approach the synthesis of complex organic compounds, which are essential in developing modern therapeutics.
Through the Pictet-Gams synthesis and his pioneering work on isoquinoline derivatives, Pictet helped lay the groundwork for modern medicinal chemistry. His legacy endures in laboratories and pharmaceutical companies worldwide, where his methods continue to enable the creation of new, effective treatments that address some of today’s most pressing health challenges.
Paul Fritsch, a significant chemist of the early 20th century, made lasting contributions to organic chemistry, especially through the development of the Pomeranz-Fritsch reaction. Born in the 19th century, Fritsch focused his work on studying and synthesizing complex molecules, particularly heterocycles. His contributions were crucial both for theoretical chemistry and for practical applications that continue to have relevance today.
Fritsch pursued his education during a period of rapid chemical discoveries and quickly gained recognition for his outstanding research. Throughout his career as a researcher and educator, he published numerous works that advanced knowledge in chemical synthesis. Although he may not have received as much recognition as some of his more famous contemporaries, his influence on the scientific community is undeniable. One of the key achievements of his career was the co-development of the Pomeranz-Fritsch reaction with Karl Pomeranz, which provided a straightforward method for synthesizing isoquinoline derivatives, a class of molecules that play a vital role in pharmaceutical research.
The Pomeranz-Fritsch reaction enables the synthesis of isoquinolines through an elegant cyclization reaction, where benzaldehyde reacts with a 2,2-dialkoxyethylamine. This reaction leads to the formation of an isoquinoline derivative. It is particularly versatile, as it is not limited to a specific diethylacetal but can also be performed with other 2,2-dialkoxyethylamines. This method allows for the relatively efficient synthesis of isoquinoline derivatives, opening a simple route to complex heterocyclic structures that serve as a foundation for drug development. Isoquinolines and their derivatives are aromatic heterocycles highly valued in pharmaceutical chemistry due to their versatility and biological activity. They often serve as scaffold structures for many drugs and play a crucial role in the development of medications.
The importance of isoquinoline derivatives in the pharmaceutical industry is immense. Many drugs used to treat malaria, hypertension, and even cancer are based on isoquinoline structures. Their ability to interact with various biological targets makes them indispensable for developing new drugs. Isoquinoline derivatives are found in pharmaceutical compounds used to treat pain, infections, cardiovascular diseases, and neurological disorders.
The Pomeranz-Fritsch reaction not only simplifies the production of these derivatives but also allows chemists to modify the structure of isoquinoline scaffolds, leading to the development of new, more effective, and safer substances. The reaction has paved the way for countless innovations in organic synthesis and has provided the foundation for discovering many new therapeutic agents. Even today, Fritsch's work continues to inspire chemists worldwide to explore further applications of this reaction, finding new solutions to global health challenges.
Fritsch's legacy is evident in the continued relevance of the reaction he co-developed and the advancements in modern drug development it enabled. Isoquinoline derivatives remain a vital component of medicinal chemistry, and the Pomeranz-Fritsch reaction remains a fundamental tool in organic synthesis. In the hands of today's scientists, Fritsch's work continues to contribute to therapeutic innovations that improve human health.
Robert Pschorr, born on March 13, 1868, in Munich, Germany, was a distinguished chemist whose contributions to the field of organic chemistry remain influential. He pursued his studies at the University of Munich, where he was mentored by the eminent chemist Adolf von Baeyer, a Nobel Laureate.
Pschorr's work primarily focused on the synthesis and structural analysis of complex organic molecules. He made significant strides in the realm of aromatic compounds, which are crucial in the development of dyes, pharmaceuticals, and many other chemical products.
Among his numerous contributions, the Pschorr cyclization stands out as a landmark achievement. This reaction is an intramolecular substitution process that involves aryldiazonium salts. In the presence of a copper catalyst and nitrous acid, these salts undergo a cyclization reaction to form polycyclic aromatic compounds. This method has been instrumental in synthesizing various complex molecules in organic chemistry, particularly in the pharmaceutical industry.
Robert Pschorr's influence extended beyond his own research. Another significant mentor in his academic journey was Ludwig Knorr, a renowned chemist known for his pioneering work in heterocyclic chemistry. Knorr's mentorship greatly influenced Pschorr's approach to organic synthesis and helped shape his innovative thinking in the field.
Robert Pschorr's innovative approaches and his mentorship under chemists like Ludwig Knorr and Adolf von Baeyer have left an indelible mark on the field of organic chemistry. His pioneering work on the Pschorr cyclization continues to be a vital tool for chemists around the world, showcasing the lasting relevance of his scientific contributions.
Bronisław Leonard Radziszewski was born in Warsaw in 1838. In 1855, he completed his secondary education in Warsaw and subsequently began studying natural sciences in Moscow. After his studies, in 1862, he became a natural science teacher at the III. Gymnasium in Warsaw. He actively participated in the January Uprising of 1863. After the uprising's failure, he left Poland to study chemistry under August Kekulé in Ghent between 1864 and 1867. He earned his doctorate in 1867 and then served as a chemistry assistant at the University of Leuven until 1870. After his time in Belgium, he returned to Poland and became the deputy professor of chemistry at the Technical Institute in Krakow. Additionally, he worked as a teacher at the secondary school. In 1872, he became a professor of general and pharmaceutical chemistry in Lwów, thus becoming the first Polish-speaking chemistry professor there. He also chaired the Department of Pharmacy and served as the director of the Chemical Institute. In 1874, he co-founded the Polish Society of Naturalists Copernicus. Starting from 1879, Radziszewski served as the Dean of the Faculty of Philosophy and, from 1882, as the Rector of the University of Lwów.
The Radziszewski synthesis is used to produce imidazoles. Imidazole is a structural element of the hormone histamine. Nowadays, histamine can also be synthetically produced by decarboxylation of the amino acid histidine. Histamine is used, among other things, in a medication for treating rare forms of leukemia and in allergy tests. To be used in medications, histamine must be produced under strict Good Manufacturing Practice (GMP) conditions.
Robert Robinson was a British chemist, born on September 13, 1886, in Derbyshire. He held professorships at several universities, including Manchester and Oxford, and was awarded the Nobel Prize in Chemistry in 1947 for his work on the structure of alkaloids and dyes. He is best known for the reaction named after him, the Robinson annulation – a process that enables the construction of cyclic structures through a combination of Michael addition and intramolecular aldol condensation. This reaction is fundamental to the total synthesis of complex natural products such as steroids. At ChemCon, the Robinson annulation is applied in the development of complex molecular frameworks, for example in the synthesis of cyclic pharmaceutical intermediates or bioactive lead structures.
The Life and Legacy of Leopold Ružička: Pioneering the Frontiers of Chemistry
In the annals of scientific achievement, few have left an indelible mark as profound as Leopold Ružička, a visionary whose work not only advanced the field of chemistry but also laid the groundwork for countless innovations in medicine, perfumery, and beyond. Born on September 13, 1887, in Vukovar, then part of the Austro-Hungarian Empire (now Croatia), Ružička's journey from a small town to the pinnacle of scientific recognition is a testament to the enduring power of curiosity and perseverance.
Early Life and Education
Ružička's academic path began at the Technical University of Karlsruhe, where he studied chemical engineering. However, it was his transition to the University of Zurich for his doctoral studies under the mentorship of Professor Hermann Staudinger that set the stage for his groundbreaking work. Staudinger, a Nobel Laureate himself, instilled in Ružička a passion for organic chemistry, particularly in the study of natural compounds.
Academic Achievements and Research
Ružička's career was distinguished by his pioneering research in the chemistry of natural substances. His early work involved the study of terpenes, a class of organic compounds found in essential oils and plant resins, which are crucial for the fragrance and flavoring industries. Ružička's investigations into the structure and synthesis of terpenes laid the foundation for modern organic synthesis, enabling chemists to create complex organic molecules in the lab.
Perhaps Ružička's most significant contribution was his research on the sex hormones. In the 1930s, he synthesized several important hormones, including testosterone and androsterone, which revolutionized the study of biochemistry and endocrinology. His work not only advanced our understanding of human biology but also had practical applications in medicine, such as in the treatment of hormonal imbalances and in the development of contraceptives.
Nobel Prize and Influence on Modern Chemistry
In recognition of his contributions to the study of organic compounds, Ružička was awarded the Nobel Prize in Chemistry in 1939 "for his work on polymethylenes and higher terpenes." This accolade was a culmination of years of meticulous research and innovation, highlighting his role in expanding the boundaries of organic chemistry.
Ružička's influence on modern chemistry extends beyond his Nobel Prize-winning work. He was a pioneer in the concept of chemical synthesis, demonstrating that it was possible to replicate and modify complex natural molecules in the laboratory. This principle has become a cornerstone of modern pharmaceutical chemistry, where synthesis plays a critical role in drug development.
Legacy and Impact
Beyond his scientific achievements, Ružička was also a mentor to future generations of chemists. As a professor at the Swiss Federal Institute of Technology in Zurich, he inspired countless students with his dedication to research and innovation. His legacy is not just in the molecules he discovered or synthesized, but also in the minds he shaped and the scientific community he helped foster.
Leopold Ružička's contributions to chemistry were transformative. By unraveling the complexities of organic molecules, he not only advanced the field of chemistry but also had a profound impact on medicine, industry, and agriculture. His work exemplifies the power of scientific inquiry to unlock the mysteries of the natural world and improve human life. As we continue to explore the frontiers of science, Ružička's pioneering spirit remains a guiding light, reminding us of the endless possibilities that await in the quest for knowledge.
Roland Scholl, a name synonymous with a pivotal advancement in organic chemistry, was a distinguished chemist whose contributions have left an indelible mark on the field. Born in the early 20th century, Scholl's journey into the realm of chemistry was characterized by an unyielding curiosity and a deep commitment to scientific discovery. His academic career was distinguished by a series of groundbreaking achievements that significantly shaped modern organic chemistry.
Scholl's most notable contribution to the field is the Scholl reaction, a coupling reaction that has become a cornerstone in the synthesis of aromatic compounds. This reaction, which he developed in the mid-20th century, involves the use of catalytic amounts of Lewis acids to facilitate the coupling of aromatic compounds. The Scholl reaction is celebrated for its ability to generate complex aromatic systems from simpler starting materials, making it a valuable tool in both research and industrial applications.
The mechanism behind the Scholl reaction is both elegant and efficient. By leveraging the unique reactivity of Lewis acids, Scholl's method allows for the formation of carbon-carbon bonds between aromatic rings, leading to the creation of new, structurally diverse aromatic compounds. This approach has proven to be instrumental in the development of various chemical products, including dyes, pharmaceuticals, and advanced materials.
Scholl's work was not only innovative in its chemical approach but also instrumental in advancing the understanding of aromatic chemistry. His research provided new insights into the behavior of aromatic systems, which has had far-reaching implications in both theoretical and applied chemistry. The Scholl reaction exemplifies how fundamental scientific research can lead to practical applications that drive industry and technological progress.
The legacy of Roland Scholl extends beyond his individual achievements. His work has paved the way for further developments in chemical synthesis and has inspired countless chemists to explore and expand upon his discoveries. The principles underlying the Scholl reaction continue to be relevant in contemporary research, and the reaction itself remains a critical tool in the arsenal of organic chemists.
In the broader context of the chemical industry, Scholl's contributions have facilitated advancements in the production of a wide range of chemical products. The ability to efficiently synthesize complex aromatic compounds has enabled the development of new materials and technologies that impact various industries, from pharmaceuticals to materials science.
Roland Scholl's legacy is a testament to the profound impact that a dedicated scientist can have on both their field and the world at large. His innovative approach to chemistry has not only enriched our understanding of aromatic compounds but has also provided practical tools that continue to drive progress in the chemical industry. As we reflect on his contributions, we are reminded of the enduring influence of his work and the ongoing relevance of his discoveries in shaping the future of chemistry.
Barry Sharpless studied at Darthmouth College. In 1968 he earned his Ph.D. in Organic Chemistry at Stanford University.
Together with William S. Knowles and Ryoji Noyori, Sharpless received the Nobel Prize in Chemistry for their work on stereoselective oxidation reactions in 2001. 21 years later, in 2022, he received his second Nobel Prize in Chemistry (for fundamental work in click chemistry) together with Carolyn Bertozzi and Morten Meldal. This makes him, besides Frederick Sanger, the only person who has been awarded with the Nobel Prize in Chemistry twice.
The Smiles rearrangement is named after British chemist Samuel Smiles, who described this reaction in the 1930s. It is a type of nucleophilic aromatic substitution that enables the intramolecular migration of substituents, typically forming new C–C or C–N bonds within aromatic systems. The Smiles rearrangement is especially useful for the targeted modification of complex aromatic molecules and the introduction of functional groups. At ChemCon, the Smiles rearrangement is used in the synthesis of functionalized aromatic building blocks, serving as intermediates for pharmaceuticals or diagnostic agents.
Kenkichi Sonogashira was a Japanese chemist whose groundbreaking work has had a lasting impact on organic chemistry. Born in Kyoto in 1931, he displayed an exceptional talent for the natural sciences early on. After studying chemistry at Kyoto University, he dedicated himself to organic synthesis, a field that was undergoing significant advancements at the time. Sonogashira aimed to develop reactions that were more efficient, environmentally friendly, and versatile. In the 1970s, he made a discovery that would make his name known worldwide: the reaction that now bears his name, the Sonogashira coupling.
This reaction, a cross-coupling between aryl or vinyl halides and terminal alkynes, opened up new possibilities in the synthesis of organic molecules. It uses a palladium catalyst in combination with a copper(I) co-catalyst and is remarkable for its efficiency and ability to proceed under mild conditions. Particularly revolutionary was its ability to easily establish C-C triple bonds, which had previously been a major challenge in organic synthesis. The Sonogashira coupling quickly found applications in the creation of complex molecules, including natural products, functional materials, and pharmaceutical compounds.
In modern research, the Sonogashira coupling has been further optimized. Scientists have developed variants that eliminate the need for copper, thereby reducing the formation of undesirable by-products such as homocoupling products. Additionally, more environmentally friendly catalytic systems have been explored, aiming to make the reaction less toxic and more cost-effective. Recent studies have demonstrated that the reaction can also be performed in aqueous media or under microwave-assisted conditions, further enhancing its sustainability. Researchers are also working to improve its efficiency in industrial processes, for example, by utilizing continuous-flow reactors.
The importance of the Sonogashira coupling for the pharmaceutical industry cannot be overstated. Many drugs contain structural motifs that can be accessed through this reaction, such as alkynes or conjugated systems. It is regularly used in the synthesis of cancer drugs, antiviral agents, and diagnostic compounds. Furthermore, the reaction is indispensable in materials science, particularly for producing organic light-emitting diodes (OLEDs) and conductive polymers, which are crucial for modern electronics.
Kenkichi Sonogashira’s reaction has laid a foundation that extends far beyond his original discovery. His legacy demonstrates how a single chemical transformation can change the way we design and utilize molecules. To this day, his work inspires chemists worldwide to explore new avenues in organic synthesis and push the boundaries of what is possible.
Hermann Staudinger, a pioneering German chemist and Nobel laureate, left an indelible mark on the field of chemistry through his groundbreaking work on macromolecules and the development of the Staudinger reaction. Born on March 23, 1881, in Worms, Germany, Staudinger's academic journey began with his studies in chemistry at the universities of Halle, Darmstadt, and Munich. After completing his doctorate in 1903 under the guidance of Daniel Vorländer, Staudinger embarked on a career that would eventually revolutionize our understanding of large molecules, now known as polymers.
Staudinger's early academic achievements were significant, though they did not immediately hint at the revolutionary path he would take. He initially focused on organic chemistry, contributing to the understanding of ketenes and their reactivity. This work laid the foundation for his later discoveries, demonstrating his keen ability to explore uncharted territories in chemistry. In 1919, while working at the Technical University of Karlsruhe, Staudinger discovered what would later be known as the Staudinger reaction, a method for synthesizing primary amines from azides. This reaction, which involves the reduction of azides by triphenylphosphine to form an iminophosphorane intermediate, was a notable advancement in organic synthesis and showcased Staudinger's innovative approach to chemical reactions.
However, it was Staudinger's work on macromolecules that truly set him apart. In the early 1920s, while serving as a professor at the University of Zurich, Staudinger began to challenge the prevailing notion that polymers were merely aggregates of small molecules held together by weak intermolecular forces. He proposed that polymers were instead long chains of covalently bonded atoms, a concept that was initially met with skepticism by the scientific community. Staudinger's persistence and dedication to proving his theory eventually led to the acceptance of the macromolecular concept, fundamentally changing the understanding of materials such as rubber, plastics, and fibers.
In 1926, Staudinger accepted a position at the University of Freiburg in Breisgau, where he would spend the remainder of his career. His time in Freiburg was marked by prolific research and numerous publications that further cemented his reputation as a leading chemist. It was during this period that Staudinger made his most significant contributions to the study of polymers. He and his colleagues conducted experiments that demonstrated the high molecular weight of these substances, providing the empirical evidence needed to support his macromolecular theory. This work laid the groundwork for the development of polymer chemistry as a distinct field of study and had far-reaching implications for industries ranging from textiles to pharmaceuticals.
Staudinger's contributions to chemistry were formally recognized in 1953 when he was awarded the Nobel Prize in Chemistry "for his discoveries in the field of macromolecular chemistry." This accolade was a testament to his visionary approach and the impact of his work on both science and industry. Even beyond his Nobel Prize-winning research, Staudinger was known for his dedication to education and his ability to inspire the next generation of chemists. His tenure in Freiburg was not only a period of intense scientific activity but also one of mentorship, as he guided many young scientists in their careers.
Hermann Staudinger passed away on September 8, 1965, but his legacy lives on through the countless applications of polymer chemistry in everyday life. From the plastic materials that are ubiquitous in modern society to the synthetic fibers used in clothing and the biopolymers essential in medical research, Staudinger's work continues to influence and shape the world. His contributions to the understanding of macromolecules and the development of synthetic methods like the Staudinger reaction have left an enduring mark on the field of chemistry, ensuring that his name will be remembered as one of the great pioneers of the 20th century.
Organic chemistry owes much to Wolfgang Steglich – not just a name, but a pioneer in natural product chemistry and synthetic methodology. Born in 1933 in Kamenz, Germany, Steglich studied chemistry in Munich, where he also earned his doctorate. After academic positions in Göttingen and Bonn, he became a professor at the University of Bonn and later at LMU Munich.
His research focused on natural products – their isolation, structural elucidation, and synthesis. However, Steglich is perhaps best known for the Steglich esterification, a mild and efficient method to form esters from carboxylic acids and alcohols using DCC and the catalyst DMAP.
This reaction quickly became a staple in organic synthesis, especially for substrates that are sensitive or sterically hindered. The reaction helps us to reliably produce complex molecules – a perfect example of Steglich's legacy: scientifically elegant, chemically robust.
Steglich received numerous awards for his scientific achievements, including the prestigious Emil Fischer Medal from the German Chemical Society and honorary doctorates from various institutions. His name lives on – not just in textbooks, but in daily lab work around the world, including at ChemCon.
John Kenneth Stille, a prominent American chemist, left an indelible mark on the field of organic chemistry through his groundbreaking research and contributions. Born in 1930, Stille dedicated his life to advancing scientific understanding, particularly in the realm of organic synthesis and polymer chemistry. His academic journey and professional accomplishments are a testament to his enduring legacy in modern science.
Stille pursued his undergraduate studies at the University of Arizona, where his fascination with chemistry took root. He later earned his Ph.D. from the University of Illinois under the mentorship of Reynold C. Fuson, focusing on organic chemistry. This foundational education equipped Stille with the knowledge and skills to tackle complex chemical problems, setting the stage for his future innovations.
One of Stille's most notable contributions to chemistry is the development of the Stille reaction, a palladium-catalyzed coupling reaction that enables the formation of carbon-carbon bonds. This reaction involves the coupling of an organostannane with an organic halide or pseudohalide. The Stille reaction is highly versatile and has become an essential tool in the synthesis of complex organic molecules, including pharmaceuticals, agrochemicals, and advanced materials. Its ability to facilitate the precise construction of molecular architectures has had a transformative impact on the pharmaceutical industry, enabling the development of drugs with improved efficacy and reduced side effects.
Beyond the Stille reaction, John Kenneth Stille made significant contributions to the field of organic polymers. His research explored the synthesis and properties of high-performance polymers, materials characterized by exceptional strength, thermal stability, and chemical resistance. These polymers have found applications in diverse industries, from aerospace to electronics, demonstrating the practical importance of Stille's work. By elucidating the fundamental principles governing polymer synthesis and behavior, he paved the way for innovations in materials science that continue to shape modern technology.
Stille's achievements resonate deeply within the pharmaceutical industry, where his work has facilitated the synthesis of complex drug molecules. The ability to construct intricate molecular frameworks with precision is crucial in drug discovery and development. The methodologies he developed have enabled chemists to design and produce compounds that target diseases more effectively, revolutionizing the treatment of conditions ranging from cancer to infectious diseases. Moreover, his contributions to polymer science have influenced the development of drug delivery systems, including biodegradable polymers and nanoparticle-based therapies, which are critical for advancing personalized medicine.
John Kenneth Stille’s legacy is not only defined by his scientific achievements but also by his dedication to education and mentorship. As a professor at Colorado State University, he inspired generations of chemists to pursue excellence in research and innovation. His influence extends far beyond the laboratory, as his discoveries continue to underpin advancements in chemistry and related fields.
In conclusion, John Kenneth Stille’s life and work exemplify the profound impact that one individual can have on science and society. His pioneering research in organic synthesis and polymer chemistry has not only expanded the frontiers of knowledge but also laid the groundwork for practical applications that benefit humanity. The Stille reaction and his contributions to polymer science remain cornerstones of modern chemistry, ensuring that his legacy endures for generations to come.
Adolph Strecker, born on October 21, 1822, in Darmstadt, Germany, is celebrated as one of the most influential chemists of the 19th century. His contributions to organic chemistry, particularly in the synthesis of amino acids, laid critical groundwork for the fields of biochemistry and pharmaceuticals. Strecker’s academic journey began with his studies at the University of Giessen, where he was mentored by Justus von Liebig, one of the most prominent chemists of the time. This mentorship undoubtedly shaped Strecker’s scientific approach, fostering a meticulous and innovative mindset that would later define his career.
Strecker is best known for the discovery of the reaction that bears his name: the Strecker synthesis. This reaction, first described in 1850, is a groundbreaking method for synthesizing α-amino acids, the fundamental building blocks of proteins. In his original experiments, Strecker demonstrated that aldehydes, when reacted with ammonia and hydrogen cyanide, could form α-amino nitriles, which could then be hydrolyzed to yield α-amino carboxylic acids. This simple yet elegant process provided a way to create amino acids in the laboratory, a remarkable achievement in an era when the molecular foundations of life were only beginning to be understood.
The significance of the Strecker synthesis extends far beyond its historical context. In modern times, the reaction has been adapted and refined to meet the needs of pharmaceutical and biochemical research. One of the most important advancements has been the development of enantioselective variants of the Strecker synthesis. These variants allow for the production of chiral amino acids with high stereoselectivity, which is crucial for pharmaceutical applications. Many drugs and biologically active molecules are chiral, and the specific enantiomer often determines the efficacy and safety of a medication. The ability to selectively produce the desired enantiomer using modified Strecker-type reactions has become a cornerstone of green and efficient pharmaceutical synthesis.
Strecker’s influence on modern chemistry and the pharmaceutical industry is profound. His work not only provided a practical method for amino acid synthesis but also inspired subsequent generations of chemists to explore the vast possibilities of organic synthesis. The principles underlying the Strecker reaction continue to inform the design of new synthetic pathways, particularly in the context of drug development. Amino acids synthesized through Strecker-inspired methods are used in the production of peptide-based drugs, nutritional supplements, and a variety of biotechnological applications.
Adolph Strecker’s legacy is one of innovation and enduring impact. His contributions to chemistry bridged the gap between theoretical understanding and practical application, providing tools and insights that are as relevant today as they were in the 19th century. Strecker passed away on November 7, 1871, but his work continues to resonate, shaping the fields of organic chemistry and pharmaceuticals in ways he could scarcely have imagined.
Akira Suzuki was born on September 12th 1930 in Mukawa, Hokkaidō prefecture, Japan.
In 1959 he received his doctorate from Hokkaidō University and became an assistant professor there two years later. From 1963 to 1965, he worked under Nobel Laureate Herbert Charles Brown at Purdue University as a postdoctoral fellow. From 1973, he worked as a professor at Japanese universities; first in the department of Applied Chemistry at Hokkaidō University, then at Okayama University and from 1995 at Kurashiki University. He also held visiting professorships at the University of Wales and Purdue University.
He conducted research in the field of organic chemistry, more specifically investigating organoboron compounds and their applications in synthesis and organometallic chemistry. With Brown he worked on hydroboration and organoborane-based organic radicals. He introduced organoboron compounds as carbanions into chemical synthesis. Later he focused on palladium catalyzed cross couplings of organoboron compounds and discovered the Suzuki Coupling.
For this discovery, he was awarded the Nobel Prize in Chemistry in 2010, together with
Ei-Ichi Negishi and Richard F. Heck.
In 2023 the Nobel Prize in chemistry went to Moungi Bawendi, Alexei Iwanowitsch Jekimow and Louis Brus. They discovered and developed quantum dots. These are of interest for multiple applications, such as:
- Marker dyes
- LEDs
- Quantumdot lasers
- Quantum computing
- And many more!
Daniel Swern was a renowned American chemist who lived from 1916 to 1982. He gained prominence for his significant contributions to organic chemistry. One of his outstanding achievements is the development of the Swern oxidation, named after him. This named reaction, also known as the Swern rearrangement, enables the mild and selective oxidation of secondary alcohols to ketones without affecting primary alcohols. The Swern oxidation is widely employed in organic synthesis worldwide and has proven to be an extremely useful tool for converting alcohols to ketones without the need for strong oxidizing conditions.
Daniel Swern's dedication to research and his innovative contributions to organic chemistry have allowed his influence to persist beyond his death. The Swern oxidation has become an integral part of the toolkit for organic chemists who synthesize complex molecules while delicately manipulating sensitive structures. Swern's legacy lives on not only in his own academic career but also in the daily work of chemists globally who benefit from his developments.
The Swern reaction finds application in the following areas:
- Pharmaceutical Industry: In the production of pharmaceutical active ingredients, alcohols can be converted to ketone structures through Swern oxidation. This can be a crucial step in the synthesis of pharmaceuticals.
- Fine Chemicals Production: In the production of fine chemicals used in various industries, the Swern reaction can be employed to generate specific ketone structures.
- Aroma Chemicals: In the aroma chemicals industry, particularly in the production of fragrances and flavorings, Swern oxidation can play a role in the synthesis of certain molecules.
- Materials Science: In some cases, Swern oxidation is also utilized in the production of polymers and other materials where specific ketone groups need to be introduced.
Frederick Nye Tebbe…
… was an organometallic chemist who published the so called Tebbe’s reagent which is usable to introduce a methylen group instead of a carbonyl functionality.
Tebbe was born in Oakland, California. He studied chemistry and received a bachelor's degree at Pennsylvania State University.
After the chemistry studies he went at Montana State University studying psychology and philosophy for a year. In 1965 he was hired by DuPont Central Research Department, where he developed Tebbe’s reagent which was named in that way by Robert Grubbs (Nobel Prize 2005).
Alfred Einhorn: A Pioneer of Chemistry and the Tscherniak-Einhorn Reaction
In the annals of chemistry, there are names whose groundbreaking discoveries and contributions to science have secured their places in history. Alfred Einhorn is undoubtedly one of these towering figures. His work has not only enriched the field of organic chemistry but has also significantly influenced practical applications in medicine and pharmacology. This post is dedicated to the life and legacy of Alfred Einhorn, focusing particularly on his renowned Tscherniak-Einhorn reaction.
Life and Academic Career
Born on February 27, 1856, in Hamburg, Alfred Einhorn's academic journey into the world of chemistry began at the University of Munich, where he studied under Adolf von Baeyer, one of the most eminent chemists of his time. Einhorn's scientific curiosity and his profound understanding of organic compounds led to numerous discoveries that defined his career.
Influential Contributions to Chemistry
Einhorn's research interests were diverse, yet his work in the field of local anesthetics stands out. His most famous contribution to chemistry and medicine was the synthesis of procaine in 1905, better known by its trade name Novocain. This discovery revolutionized surgical medicine by providing an effective and less toxic alternative to the local anesthetics used at the time.
The Tscherniak-Einhorn Reaction
Another significant achievement of Einhorn's was the development of the Tscherniak-Einhorn reaction, named after him and his colleague Tscherniak. This chemical reaction expanded the understanding of organic compound synthesis and had a profound impact on organic chemistry. The Tscherniak-Einhorn reaction facilitates the synthesis of α-hydroxyphosphonates, which play a crucial role as key intermediates in organic synthesis and pharmaceutical development.
Collaboration and Influence
Alfred Einhorn's work was characterized by collaboration with other scientists and by his ambition to push the boundaries of chemical knowledge of his time. His influence on subsequent generations of chemists and his contributions to organic synthesis and pharmaceutical chemistry are immeasurable. His research laid the foundation for modern anesthesia methods and improved the quality of life for millions of patients worldwide.
Legacy and Modern Science
Alfred Einhorn's legacy extends far beyond his lifetime. The methods and compounds he developed continue to form the basis for scientific research and medical practice today. The Tscherniak-Einhorn reaction and the synthesis of Novocain are just two examples of Einhorn's groundbreaking work that have permanently transformed chemistry and medicine.
Conclusion
Alfred Einhorn was a visionary scientist whose curiosity and spirit of innovation expanded the boundaries of understanding in chemistry. His discoveries, especially the Tscherniak-Einhorn reaction and the synthesis of Novocain, have made him a key figure in the development of modern chemistry and pharmacology. His legacy lives on in every application of his research, a lasting testament to the power of science to improve human life.
Barry Trost is a renowned American chemist whose work has had a lasting impact on organic synthesis. Born in 1941, he studied at the University of Pennsylvania and later earned his Ph.D. at the Massachusetts Institute of Technology (MIT). After working at the University of Wisconsin-Madison, he joined Stanford University, where he remains active today.
Trost is known for his contributions to atom-economical synthesis, a principle that optimizes chemical reactions to minimize waste. One of his most significant discoveries is the Tsuji-Trost reaction, named after him and Tamejiro Tsuji. This palladium-catalyzed allylation allows for the precise introduction of functional groups into organic molecules and is widely used in the synthesis of natural products, pharmaceuticals, and complex organic structures. It has revolutionized modern synthesis strategies by offering high selectivity and efficiency.
Throughout his career, Trost has received numerous prestigious awards, including the ACS Awards in Organic Chemistry and the Wolf Prize. His research has not only shaped scientific advancements but has also found practical applications in industry.
ChemCon utilizes the Tsuji-Trost reaction in its custom synthesis services to produce tailored organic compounds for pharmaceutical and biotechnological applications. This efficient method enables the synthesis of high-purity intermediates with excellent selectivity and atom-economic principles, contributing to sustainability and quality assurance.
Ivar Ugi, born in 1930 in Kuressaare, Estonia, was a prominent chemist whose work revolutionized organic synthesis. After World War II, he emigrated to Germany, where he began his academic career. He studied chemistry at the University of Tübingen and earned his Ph.D. in 1954 under the supervision of the renowned chemist Rolf Huisgen. In the 1960s, Ugi developed the reaction that bears his name, the Ugi reaction, a multicomponent reaction that continues to have a significant impact on chemical research and industry today.
Ugi was highly successful in his academic career, holding various professorships, including in Munich. He worked at prestigious research institutes and made important contributions to chemistry, particularly in the field of organic synthesis. His research led to numerous publications and patents that fundamentally changed the way chemists synthesize molecules and pharmaceutical compounds.
The Ugi reaction is one of the most well-known multicomponent reactions, in which a ketone or aldehyde, an amine, an isonitrile, and a carboxylic acid are combined in a single step to form a bis-amide. This reaction is not only efficient but also allows for great variability in the selection of reagents, making it a valuable tool in pharmaceutical chemistry. The fast and simple execution of the Ugi reaction has made it a preferred method in drug development.
A standout example of the Ugi reaction’s significance is its role in the synthesis of Indinavir, a drug used in the treatment of HIV. Indinavir is a protease inhibitor that is employed in HIV therapy to block the virus’s replication. The Ugi reaction enabled the efficient and scalable synthesis of Indinavir, playing a key role in the development of this life-saving medication.
The importance of Ugi’s work in HIV therapy cannot be overstated. By applying the Ugi reaction, complex drugs like Indinavir could be synthesized more quickly and efficiently. This contributed to improving treatment options for millions of HIV patients worldwide. Ivar Ugi’s discovery not only advanced the field of chemistry but also made a lasting contribution to medical research and the fight against one of the most devastating epidemics of our time.
Fritz Ullmann was a German chemist.
He studied at the University of Geneva, where he also received his PhD in 1895. At the Technische Hochschule Berlin he lectured technical chemistry from 1905 to 1913 as private lecturer and from 1922 to 1925 as an associated professor.
From 1914 to 1922, he published the first edition of the Encyclopedia of Industrial Chemistry in 12 volumes, under the name “Encyclopedia of Industrial Chemistry” a standard work that is still constantly updated.
He discovered some important preparative synthesis methods like the synthesis of diarylamines, synthesis of carbazoles and of course the Ullmann reaction, which we present you here.
Franz Varrentrapp was a German chemist of the 19th century whose contributions to analytical chemistry are primarily known today through the reaction that bears his name: the Varrentrapp reaction. Born on November 18, 1815, in Braunschweig, he studied natural sciences, including chemistry, in Göttingen. Later, he worked closely with Justus von Liebig, one of the most influential chemists of the era, which significantly shaped his scientific career. Varrentrapp focused particularly on the development of methods for detecting and quantifying chemical elements in organic compounds — a central need during a period of rapid advancement in organic chemistry. His work contributed greatly to the standardization of analytical procedures and laid the foundation for modern elemental analysis techniques.
The Varrentrapp reaction is a classical method for detecting and quantitatively determining sulfur in organic compounds. The organic sample is heated with sodium, converting sulfur into sodium sulfide, which can then be detected through precipitation as lead(II) sulfide. While modern analytical techniques have largely replaced this method, it remains a historically significant example of early quantitative elemental analysis. At ChemCon, classical methods like the Varrentrapp reaction are still occasionally employed in method development and validation processes, especially when robust and traceable detection of regulatory-relevant elements such as sulfur is required.
Anton Vilsmeier was a German chemist whose contribution to organic chemistry was profoundly shaped by the reaction named after him, the Vilsmeier-Haack reaction. Born on June 12, 1894, in Burgweinting, now a district of Regensburg, Vilsmeier showed an early interest in the natural sciences. After graduating from high school, he began studying chemistry at the University of Munich in 1920 and continued his education at the University of Erlangen from 1922 onwards. There, he earned his doctorate in 1924 under the mentorship of Otto Fischer with a dissertation on gamma-chloro-iso-chinocyanines derived from methyl-(ethyl-)acetanilide and phosphorus oxychloride.
During his time in Erlangen, Vilsmeier closely collaborated with Albrecht Haack. Together, they developed the Vilsmeier-Haack reaction in 1927, a method for formylating aromatic and heterocyclic compounds using dimethylformamide (DMF) and phosphorus oxychloride, yielding aryl and heteroaryl aldehydes. This method proved to be extremely efficient and versatile, quickly gaining widespread application in the synthesis of intermediates for dyes and pharmaceuticals.
In 1927, Vilsmeier took up a position at BASF in Ludwigshafen, where he worked until his retirement in 1959. During his industrial career, he focused on developing vat dyes of the Indanthrene series, significantly contributing to advancements in dye chemistry. His work not only impacted the academic world but also had a lasting influence on the chemical industry. The ability to selectively formylate aromatics enabled the synthesis of complex molecules used as building blocks in pharmaceutical drug development. Many pharmaceutical compounds are based on aryl aldehydes, which can be efficiently synthesized using the Vilsmeier-Haack reaction.
At ChemCon, this historical reaction is combined with state-of-the-art technology to ensure the highest purity and efficiency in production. As a leading company in the field of custom synthesis, ChemCon uses the Vilsmeier-Haack reaction to develop tailor-made molecules according to the specific requirements of our clients. This flexibility allows for the synthesis of innovative active ingredients and intermediates that meet stringent GMP standards. By applying this classic yet highly relevant synthesis method, ChemCon efficiently produces complex organic compounds, contributing to the advancement of the pharmaceutical industry. Anton Vilsmeier's legacy lives on in modern chemical synthesis, illustrating the timelessness of his scientific achievement.
Julius von Braun was a German chemist who was born in Warsaw on July 26, 1875 and died in Heidelberg on January 8, 1939. After passing his school-leaving examination at the humanistic grammar school in Warsaw in 1893, he studied chemistry at the University of Göttingen, the Royal Technical University of Charlottenburg and the University of Munich. In 1898, he obtained his doctorate under Nobel Prize winner Otto Wallach at the University of Göttingen.
Braun worked as an assistant at the Institute of Chemistry in Göttingen and became a private lecturer in 1902. In 1909 he became associate professor at the University of Breslau and later professor at the Berlin Agricultural College. In 1921, he moved to the University of Frankfurt am Main as a full professor of chemistry.
Braun researched the reactions of numerous alkaloids and synthesized organic compounds. He also discovered the Rosenmund-von Braun reaction and the Von Braun reaction. The latter is also known as the von Braun amide degradation reaction and is a named reaction in organic chemistry. This synthesis produces halogenoalkanes and nitriles.
Today, haloalkanes have a variety of applications, including as solvents, coolants, propellants, flame retardants and in medicine. However, the use of haloalkanes as propellants in medicine is controversial, as they can contribute to the destruction of the ozone layer.
Georg Friedrich Karl Wittig:
Wittig was a German chemist and Nobel Prize winner. Because of his family background, he was very talented artistically. He played the piano, composed and painted very well. However, his love was chemistry. Although he was drafted in the middle of his chemistry studies and became a prisoner of war, he continued his studies as soon as he was free.
By means of the Wittig reaction, carbon-carbon double bonds can be formed. This involves the use of a carbonyl compound and a phosphonium ylide, with the carbonly oxygen substituted for the carbon.
Karl Ziegler was a German chemist known for his contributions to polymer chemistry, organometallic chemistry, and homogeneous transition metal catalysis. He was born in Helsa in 1898 and studied chemistry at the University of Marburg, where he received his doctorate in 1920. He initially worked at I.G. Farben Industrie AG before embarking on an academic career. He was a professor at the universities of Frankfurt, Heidelberg, and Tübingen before taking over as director of the Max Planck Institute for Coal Research in Mülheim an der Ruhr in 1943.
One of his most important scientific achievements was the discovery of the Ziegler-Natta catalyst, which makes it possible to produce polymers with a precise molecular structure. These polymers have improved properties and find diverse applications in the plastics industry. Ziegler also invented the Ziegler process for the production of fatty alcohols from ethene and triethylaluminium, which serve as raw materials for biodegradable detergents. In addition, he researched free radicals, alkali-organic compounds, ring-closing reactions, natural substances, and electrochemical phenomena.
For his outstanding achievements, Ziegler was awarded numerous honors, including the Nobel Prize in Chemistry in 1963, which he shared with Giulio Natta. He was also a co-founder and first president of the German Chemical Society (GDCh) and an honorary citizen of the city of Mülheim. He donated a large part of his fortune to promote chemical research and left behind a significant art collection. He died in 1973 at the age of 74.
Ludwig Wolff: A Pillar in Organic Chemistry and Pharmaceutical Innovation
In the annals of chemistry, few have left as indelible a mark as Ludwig Wolff, a German chemist whose work has resonated through the corridors of time, influencing not just academic circles but also the very fabric of the pharmaceutical industry. Born in the late 19th century, Wolff embarked on a journey that would see him delve into the complexities of organic chemistry, unearthing reactions and processes that bear his name and continue to be of paramount importance today.
Wolff's academic career was a testament to his brilliance and dedication. He was known for his rigorous approach to research and an insatiable curiosity that drove him to explore the intricate dance of atoms and molecules. His work was pioneering, laying the groundwork for future chemists to build upon. Among his numerous contributions, the Wolff rearrangement stands out as a beacon of his ingenuity.
The Wolff Rearrangement, a chemical reaction that involves the rearrangement of α-diazoketones to ketenes, is a cornerstone in synthetic organic chemistry. This process has become a vital tool for chemists, enabling the synthesis of complex molecules from simpler precursors. It's a testimony to Wolff's forward-thinking that his discovery has found applications far beyond what might have been imagined during his time, particularly in the pharmaceutical industry.
In today's pharmaceutical landscape, the Wolff rearrangement plays a critical role in the synthesis of various compounds, including antibiotics and anti-inflammatory drugs. Its ability to efficiently create ketenes, which can be readily transformed into a plethora of functional groups, makes it invaluable in the design and development of new drugs. The flexibility and versatility offered by this rearrangement have enabled chemists to innovate and create more effective and targeted therapies, improving patient outcomes and contributing to the advancement of medicine.
Moreover, Wolff's influence extends beyond the confines of his own discoveries. His dedication to the pursuit of knowledge and his innovative thinking have inspired generations of chemists. He embodies the spirit of inquiry and resilience, reminding us that the quest for understanding requires not just intelligence but also imagination and perseverance.
The legacy of Ludwig Wolff is not just etched in the pages of chemistry textbooks but is alive in the laboratories where his work continues to inspire innovation. As the pharmaceutical industry evolves, the principles and processes he pioneered remain at the heart of drug development. His contributions serve as a foundation upon which new discoveries are made, demonstrating the enduring impact of his work on the health and well-being of society.
In conclusion, Ludwig Wolff's life and academic achievements are a testament to the profound influence one individual can have on the world. The Wolff rearrangement is a reminder of his genius, a gift to the realms of chemistry and medicine that has propelled the pharmaceutical industry into new frontiers. As we look to the future, Wolff's legacy serves as both a cornerstone and a beacon, guiding ongoing efforts to unravel the mysteries of chemistry and harness them for the betterment of humanity.
Charles Adolphe Wurtz, also known as Karl Adolph Wurtz, was born on November 26, 1817, in Strasbourg and died on May 12, 1884, in Paris. He was a French physician and chemist whose main area of study was the chemistry of hydrocarbons and organic nitrogen compounds.
Wurtz was the son of Jean Jacques Wurtz, a Protestant pastor in Strasbourg and Wolfisheim, where Charles Adolphe Wurtz spent his early youth. After attending the Protestant high school in Strasbourg in 1834, he began studying medicine with his father's consent. His interest in clinical chemistry led to his appointment as head of chemical work at the Strasbourg Medical Faculty in 1839.
Wurtz synthesized ethylamine and discovered glycol and phosphoroxychloride. Along with Rudolph Fittig, the Wurtz-Fittig synthesis was named, which produces hydrocarbons from halogen alkanes by the action of alkali metals.
After a year of study under Justus von Liebig in Giessen, he returned to Paris, where he worked in the laboratory of Jean-Baptiste Dumas. In 1845, he became Dumas' assistant at the École de Médicine de Paris, and four years later, he began lecturing on organic chemistry there.
Despite the modest equipment of his laboratory at the École de Médicine de Paris, he opened his own private laboratory in 1850 on Rue Garenciere. In the same year, he was appointed Professor of Chemistry at the newly opened Institut Agronomique in Versailles, which was closed again in 1852.
The following year, he took over the chair of Organic Chemistry at the Medical Faculty, which had become vacant due to the resignation of J.B.A. Dumas. In 1866, he took on the duties of dean at the Faculty of Medicine. In 1875, he retired from the office of dean, but retained the title of Honorary Dean.
Wurtz was an honorary member of almost all scientific societies in Europe. He was one of the founders of the Société Chimique de France (1858), where he was the first secretary and served three times as president. His work and influence on chemistry are still felt today, particularly through the Wurtz reaction named after him.
The ethylamine he discovered is used in the production of many herbicides, pesticides, and in the manufacture of rubber. Ethylene glycol is used in antifreeze and the production of polyester fibers.
Masaru Yamaguchi stands as a notable figure in the field of organic chemistry, with his groundbreaking work leaving a lasting impact on the scientific community. A professor at Kyoto University, Yamaguchi's innovative approaches to synthesis have paved the way for numerous advancements in chemical research.
One of Yamaguchi’s most renowned contributions is the development of the Yamaguchi Cyclization, a method that has revolutionized the synthesis of macrocyclic lactones. This reaction involves the formation of macrocyclic rings from hydroxycarboxylic acids using Yamaguchi's unique reagents, such as 2,4,6-trichlorobenzoyl chloride (TCBC) and 4-dimethylaminopyridine (DMAP). The procedure stands out for its efficiency and high yield, enabling the creation of large, complex ring structures that are essential in various natural products and pharmaceuticals.
The Yamaguchi Cyclization is particularly significant in the synthesis of macrolides, a class of natural products with potent biological activities, including antibiotic and anticancer properties. By simplifying the formation of these intricate structures, Yamaguchi's method has become a staple in organic synthesis laboratories worldwide.
Masaru Yamaguchi's contributions extend beyond his named cyclization. His research has laid the groundwork for advancements in catalysis, green chemistry, and drug development. Yamaguchi’s work has inspired the development of new catalytic processes that improve the efficiency and selectivity of chemical reactions. Emphasizing sustainability, his methodologies often involve milder conditions and fewer byproducts, aligning with the principles of green chemistry. Additionally, the ability to efficiently synthesize complex molecules has accelerated the discovery and production of new pharmaceuticals, particularly those involving macrocyclic structures.
Today, the influence of Masaru Yamaguchi can be seen in the continued exploration of cyclization techniques and the ongoing quest for more sustainable and efficient chemical processes. Researchers build upon his pioneering work, striving to push the boundaries of what is possible in organic synthesis. Yamaguchi's dedication to innovation and excellence in chemistry serves as an enduring inspiration to chemists around the globe, ensuring that his legacy will continue to shape the field for generations to come.
Theodor Zincke was born on May 19, 1843 in Uelzen and was an influential German chemist whose work and discoveries had a significant impact on modern chemistry. Zincke studied at the University of Göttingen, where he received his doctorate in 1865. He then worked under various chemists in Germany and France, including Hermann Kolbe and Charles-Adolphe Wurtz, which helped him to deepen his knowledge and skills in organic chemistry.
His academic career began at the University of Marburg, where he was appointed Professor of Chemistry in 1872. Later, in 1875, he moved to the University of Bonn, where he worked until his retirement in 1913. In Bonn, he headed the Institute of Chemistry and carried out numerous pioneering research projects.
One of his most important scientific achievements was the development of the Zincke reaction named after him. This reaction, in which pyridines are converted into pyridinium salts, was an important contribution to organic synthesis and was widely used in the research of heterocycles. In addition, Zincke researched the chemistry of quinones and nitro compounds, which also led to important findings in organic chemistry.
Zincke's work had a lasting influence on chemical research and teaching. His studies on the structure and reactivity of organic molecules laid the foundation for many modern developments in chemistry. In particular, his work on pyridines and the reactions he developed are still relevant and are used in organic synthesis and pharmaceutical chemistry.
Theodor Zincke was also a dedicated teacher and mentor for many young chemists. Numerous doctoral theses were completed under his guidance, and many of his students went on to have significant careers in chemistry themselves. Zincke's influence on today's science can therefore be seen not only in his own discoveries, but also in the training and inspiration of the next generation of chemists.
He died on March 17, 1928, but his legacy lives on. The reactions and methods he developed are an integral part of chemical knowledge and continue to be used in research and industry. Zincke remains an outstanding example of a scientist whose work and influence extended far beyond his own lifetime.