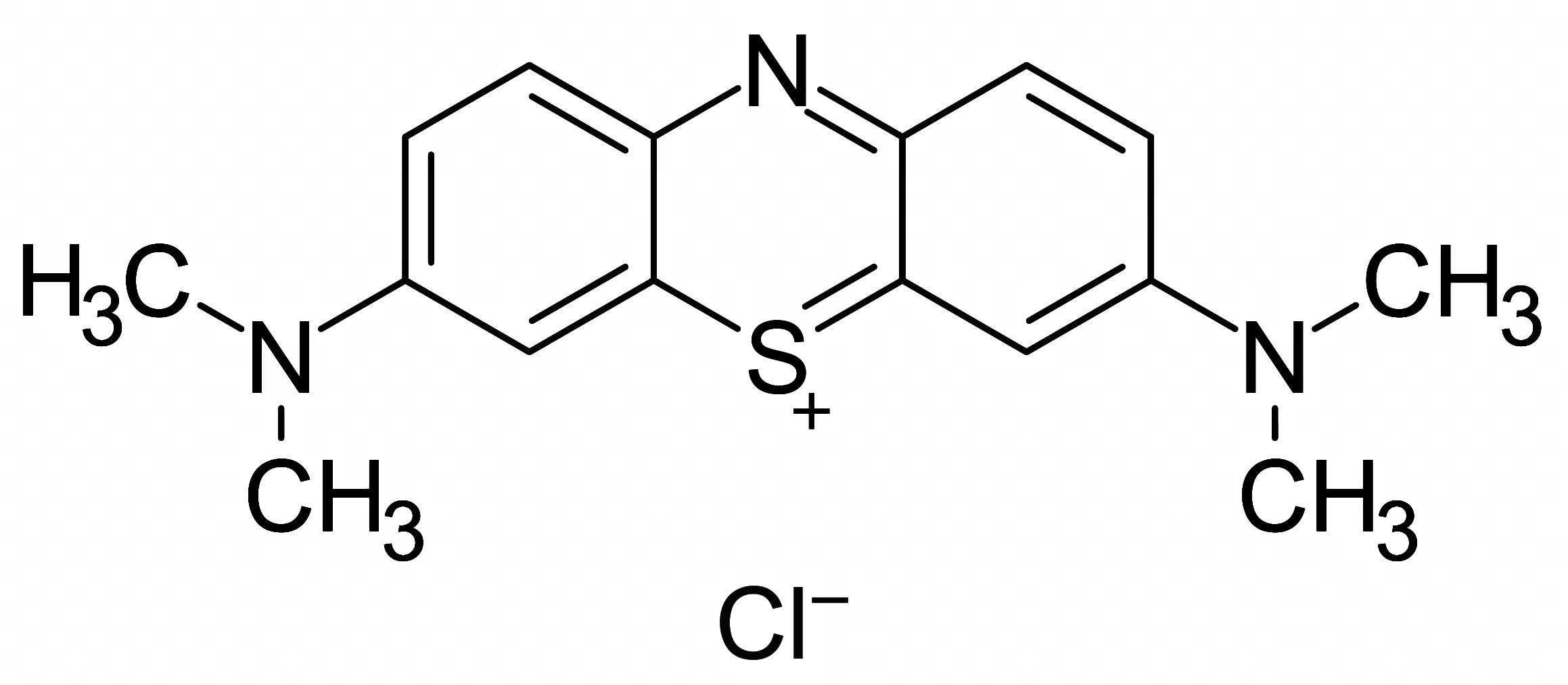
Summary
Synonyms: N,N,N′,N′-Tetramethylthionine chloride, Methylthioninium chloride, 3,7-Bis(dimethylamino)phenothiazinium chloride, C.I. Basic Blue 9
Molecular weight: 319.85 g/mol (anhydrous)
Appearance: Dark green, lustrous crystals
Melting point: approx. 190 °C
Density: 1.0 g/cm³ (at 20 °C)
Solubility: Soluble in water (20 °C: approx. 40 g/L), moderately soluble in ethanol and chloroform, practically insoluble in diethyl ether
CAS number: 122965-43-9 (monohydrate)
Methylene Blue (Methylthioninium chloride) is a chemical compound of high pharmaceutical relevance with a broad range of medical and diagnostic applications. As a well-established medicinal product, Methylene Blue is included in the WHO Model List of Essential Medicines of the World Health Organization (WHO), underlining its clinical importance and worldwide acceptance.
Due to its high purity, reliable quality, and manufacture under strict GMP conditions, Methylene Blue is particularly suitable for pharmaceutical applications, including parenteral use. Injection Grade Methylene Blue meets the increased requirements for purity, safety, and process control and is therefore used in diagnostic applications as well as in research and development.
Medical Applications
Methylene Blue (Methylthioninium chloride) is primarily used in clinical practice for the treatment of acquired methemoglobinemia. For this indication, Injection Grade Methylene Blue is required, as the highest standards for purity, control of impurities, and consistent quality must be met.
In addition, Methylene Blue is used as an antidote in selected toxicological emergencies as well as in diagnostic and clinical procedures that require parenterally applicable material.
Regulatory Status
Approved in the EU (EMA) and the USA (FDA) for the treatment of methemoglobinemia in defined indications.
Mechanism of Action
Methylene Blue (Methylthioninium chloride) acts as a redox-active electron acceptor. Following parenteral administration, it is reduced in vivo to leukomethylene blue, which supports the enzymatic conversion of methemoglobin back to functional hemoglobin.
Through this mechanism, the oxygen-carrying capacity of the blood is effectively restored. The mechanism of action is rapid in onset, well characterized, and clinically established for decades, particularly in acute and emergency medicine.
Advantages of Injection Grade Methylene Blue
Compared with alternative or supportive treatment options, Injection Grade Methylene Blue offers several key advantages:
- Direct, causal mechanism of action in the treatment of methemoglobinemia
- Rapid onset of action, particularly with intravenous administration
- High chemical and microbiological purity, suitable for parenteral use
- Extensive clinical experience and a well-documented safety profile
- Worldwide acceptance, including inclusion in the
WHO Model List of Essential Medicines - No requirement for complex adjunct therapies when used according to the approved indication
Due to these properties, Methylene Blue is regarded in many clinical guidelines as the therapy of first choice for the treatment of acquired methemoglobinemia.
ChemCon Injection Grade Methylenblau
ChemCon offers injection grade methylene blue in pharmaceutical quality, specifically developed and manufactured for parenteral applications.
Production is carried out under strict GMP conditions in our facilities certified by the local regulatory authority and the FDA. The product meets the highest requirements for purity, process control, documentation, and regulatory compliance, as required for injectable APIs.
ChemCon Quality
At ChemCon, we stand for the highest quality in the GMP manufacturing of pharmaceutical APIs, including injection grade methylene blue. Our production processes comply with the most stringent international standards to ensure that all products meet the highest requirements for parenteral and injectable applications.
We provide comprehensive GMP documentation and regulatory support, supporting our customers in meeting applicable EMA and FDA requirements. Through continuous quality control, validated processes, and the certification of our laboratories by the local regulatory authority and the FDA, we ensure that each batch of injectable methylene blue API consistently meets the highest quality, purity, and compliance standards.
Downloads
more information

ChemCon high quality APIs

ChemCon company brochure
Sources: https://meshb.nlm.nih.gov/record/ui?ui=D008751, Jan 2026
World Health Organization (WHO), WHO Model List of Essential Medicines/ CC BY-NC-SA 3.0 IGO.
Wikipedia: Methylenblau, https://de.wikipedia.org/wiki/Methylenblau, Jan 2025/ CC-BY-SA 4.0