Active pharmaceutical ingredients (APIs)
Here you will find selected examples of compounds, for which the production at ChemCon is already established or under development.
ChemCon’s APIs are released to current monographs or to defined specifications following validated internal methods. Please let us know which release specifications you require, and we will adjust our protocol accordingly.
Our APIs
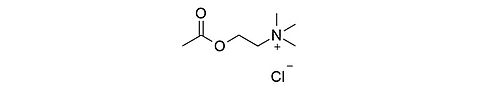
Acetylcholine chloride [60-31-1]
CAS Number: 60-31-1
Molecular weight: 181.66 g/mol
Chemical formula: C7H16ClNO2
Synonyms: 2-(Acetyloxy)-N,N,N-trimethylethanaminium chloride,
(2-Acetoxyethyl)trimethylammonium chloride
Quality: GMP, injectable / ophthalmic grade
Development stage: validated
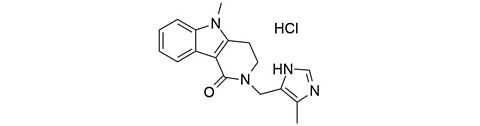
Alosetron hydrochloride [122852-69-1]
CAS Number: 122852-69-1
Molecular weight: 330.81 g/mol
Chemical formula: C17H18N4O · HCl
Synonyme: 5-Methyl-2-[(4-methyl-1H-imidazol-5-yl)methyl]-3,4-dihydro-2H-pyrido[4,3-b]indol-1(5H) hydrochloride,
1H-Pyrido(4,3-b)indol-1-one, 2,3,4,5-tetrahydro-5-methyl-2-((5-methyl-1H-imidazol-4-yl)methyl)-, monohydrochloride,
2,3,4,5-Tetrahydro-5-methyl-2-((5-methylimidazol-4-yl)methyl)-1H-pyrido(4,3-b)indol-1-one monohydrochloride
Quality: GMP
Development stage: intermediate, please contact us regarding timelines
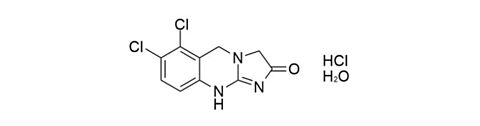
Anagrelide hydrochloride monohydrate [823178-43-4]
CAS Number: 823178-43-4
Molecular weight: 310.57 g/mol
Chemical formula: C10H7Cl2N3O · HCl · H2O
Quality: GMP, oral grade
Development stage: validated
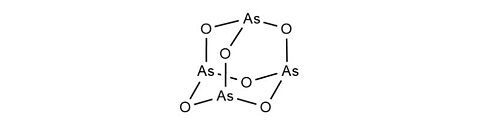
Arsenic trioxide [1327-53-3]
CAS Number: 1327-53-3
Molecular weight: 197.84 g/mol
Chemical formula: As2O3
Synonyme: Arsenic(III)-oxide, Arsenous acid anhydride
Quality: GMP, injectable / oral grade
Development stage: validated
Chromium(III) chloride hexahydrate [10060-12-5]
CAS Number: 10060-12-5
Molecular weight: 266.41 g/mol
Chemical formula: CrCl3 · 6 H2O
Synonyms: chromium trichloride, chromium chloride
Quality: GMP, injectable grade
Development stage: validated
Copper(II) chloride dihydrate [10125-13-0]
CAS Number: 10125-13-0
Molecular weight: 170.48 g/mol
Chemical formula: CuCl2 · 2 H2O
Synonyms: copper dichloride dihydrate, copper chloride dihydrate, Cupric chloride dihydrate
Quality: GMP, injectable grade
Development stage: validated
Copper(II) sulfate anhydrous [ [7758-98-7]
CAS Number: 7758-98-7
Molecular weight: 159,61 g/mol
Chemical formula: CuSO4
Synonyms: Cupric sulfate
Quality: GMP, injectable grade
Development stage: early, please contact us regarding timelines
Copper(II) sulfate pentahydrate [7758-99-8]
CAS-Nummer: 7758-99-8
Molekulargewicht: 249,69 g/mol
Summenformel: CuSO4 x 5 H2O
Synonyme: Cupric sulfate, blue vitriol
Qualität: GMP, injectable grade
Entwicklungsstand: validated
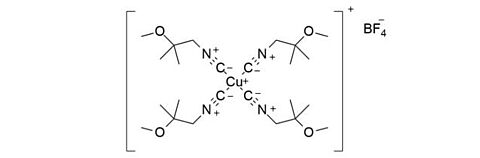
Copper tetraMIBI tetrafluoroborate [103694-84-4]
CAS Number: 103694-84-4
Molecular weight: 602.98 g/mol
Chemical formula: C24H44CuN4O4 ⋅ BF4
Synonyme: (2-Methoxy-2-methylpropaneisocyanide)copper(I) tetrafluoroborate,
MIBI copper complex, Tetrakis(2-methoxyisobutyl isonitrile)copper(I) tetrafluoroborate
Quality: GMP, for the preparation of technetium sestaMIBI
Development stage: intermediate, please contact us regarding timelines
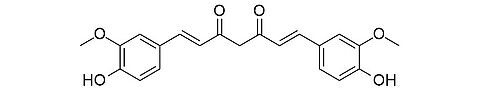
Curcumin [458-37-7]
CAS Number: 458-37-7
Molecular weight: 368.39 g/mol
Chemical formula: C21H20O6
Synonyms:1,5-Divanillyliden-2,4-pentandione,
1,7-Bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione,
Curcumin, Diferuloylmethane
Development stage: early, please contact us regarding timelines
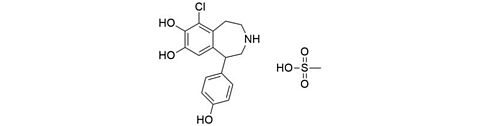
Fenoldopam mesylate [67227-57-0]
CAS Number: 67227-57-0
Molecular weight: 401,87 g/mol
Chemical formula: C16H16ClNO3 · CH3SO3H
Synonyms: 9-chloro-5-(4-hydroxyphenyl)-2,3,4,5-tetrahydro-1H-3-benzazepine-7,8-diol
Quality: GMP, injectable grade
Development stage: validated
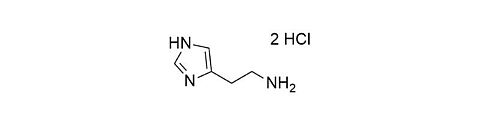
Histamine dihydrochloride [56-92-8]
CAS Number: 56-92-8
Molecular weight: 184.07 g/mol
Chemical formula: C5H9N3 · 2 HCl
Synonyms: 2-(1H-Imidazol-4-yl)ethanamine dihydrochloride,
4-(2-Aminoethyl)imidazole dihydrochloride
Quality: GMP, topic grade
Development stage: validated
Validation of further release methods for oral / injectable grade is possible upon request.
Iron(III) chloride hexahydrate [10025-77-1]
CAS Number: 10025-77-1
Molecular weight: 270.30 g/mol
Chemical formula: FeCl3 · 6 H2O
Synonyms: iron trichloride, Ferric Chloride
Quality: GMP, injectable grade
Development stage: validated
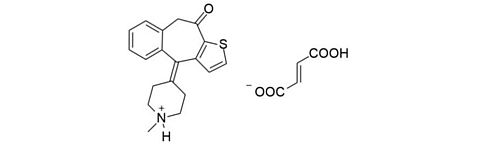
Ketotifen fumarate [34580-14-8]
CAS Number: 34580-14-8
Molecular weight: 425.50 g/mol
Chemical formula: C19H19NOS · C4H4O4
Synonyms: 4,9-Dihydro-4-(1-methyl-4-piperidinylidene)-10H-benzo(4,5)cyclohepta(1,2-b)thiophen-10-one
Quality: GMP, ophthalmic grade
Development stage: early, please contact us regarding timelines
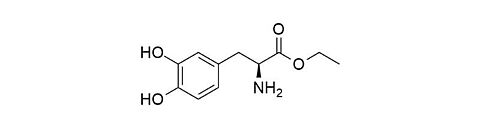
L-Dopa ethyl ester (Etilevodopa) [37178-37-3]
CAS Number: 37178-37-3
Molecular weight: 225.24 g/mol
Chemical formula: C11H15NO4
Synonyms: (-)-3-(3,4-Dihydroxyphenyl)-L-alaninethylester,
2-Amino-3-(3,4-dihydroxyphenyl)propansäurethylester,
Levodopa ethyl ester, Etilevodopa
Quality: GMP, oral grade
Development stage: intermediate, please contact us regarding timelines
Validation of further release methods for injectable grade is possible upon request.
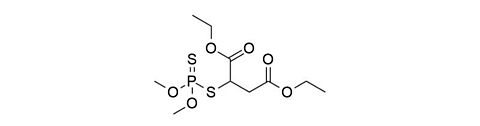
Malathion [121-75-5]
CAS Number: 121-75-5
Molecular weight: 330.36 g/mol
Chemical formula: C10H19O6PS2
Synonyms: O,O-Dimethyl S-(1,2-bis(ethoxycarbonyl)ethyl) dithiophosphate,
Butanedioic acid, ((dimethoxyphosphinothioyl)thio)-, diethyl ester
Quality: GMP, topic grade
Development stage: validated
Manganese(II) chloride tetrahydrate [13446-34-9]
CAS Number: 13446-34-9
Molecular weight: 197.91 g/mol
Chemical formula: MnCl2 · 4 H2O
Synonyms: Manganese(II) chloride, Manganese dichloride
Quality: GMP, injectable grade
Development stage: validated
Manganese(II) sulfate monohydrate [10034-96-5]
CAS Number: 10034-96-5
Molecular weight: 169.02 g/mol
Chemical formula: MnSO4 ∙ H2O
Synonyms: -
Quality: GMP, injectable grade
Development stage: validated
Melphalan free base [148-82-3]
CAS Number: 148-82-3
Molecular weight: 305.20 g/mol
Chemical formula: C13H18Cl2N2O2
Synonyms: 3-(p-(Bis(2-chloroethyl)amino)phenyl)-L-alanine,
3-p-(Di(2-chloroethyl)amino)-phenyl-L-alanine,
4-(Bis(2-chloroethyl)amino)-L-phenylalanine,
p-Di-(2-chloroethyl)amino-L-phenylalanine, L-Sarcolysin
Quality: GMP, oral grade
Development stage: validated
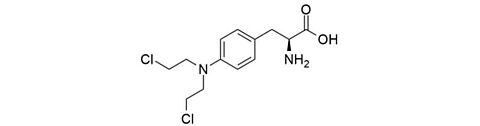
Melphalan hydrochloride [3223-07-2]
CAS Number: 3223-07-2
Molecular weight: 341.66 g/mol
Chemical formula: C13H18Cl2N2O2 · HCl
Synonyms: 3-(p-(Bis(2-chloroethyl)amino)phenyl)-L-alanine hydrochloride,
3-p-(Di(2-chloroethyl)amino)-phenyl-L-alanine hydrochloride,
4-(Bis(2-chloroethyl)amino)-L-phenylalanine hydrochloride,
p-Di-(2-chloroethyl)amino-L-phenylalanine hydrochloride,
L-Phenylalanine mustard hydrochloride
Quality: GMP, injectable grade
Development stage: validated
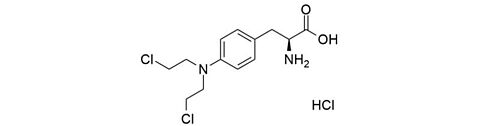
Metadoxine [74536-44-0]
CAS-Number: 74536-44-0
Molecular weight: 298.29 g/mol
Chemical formula: C13H18N2O6
Synonyms: 4,5-bis(hydroxymethyl)-2-methylpyridin-3-ol;(2S)-5-oxopyrrolidine-2-carboxylic acid
Quality: GMP oral grade
Development stage: advanced, please contact us regarding timelines
Methylene blue [122965-43-9]
CAS Number: 122965-43-9 (monohydrate)
61-73-4 (anhydrous),
7220-79-3 (trihydrate),
32680-41-4 (pentahydrate)
Molecular weight: 319.85 g/mol (anhydrous)
Chemical formula: C16H18CIN3S
Synonyms: N,N,N′,N′-Tetramethylthioninchloride,
3,7-Bis(dimethylamino)phenazathionium chloride,
Methylthionium chloride, C.I. Basic Blue 9
Quality: GMP, injectable / oral grade
Development stage: validated
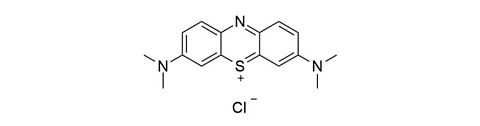
Nicardipine hydrochloride [54527-84-3]
CAS Number: 54527-84-3
Molecular weight: 515.99 g/mol
Synonyms: 2-(Benzylmethylamino)ethyl methyl 1,4-dihydro-2,6-dimethyl-4-(m-nitrophenyl)pyridine-3,5-dicarboxylat,
3,5-Pyridinedicarboxylic acid 1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)-, 2-(benzylmethylamino)ethyl methyl ester
Chemical formula: C26H29N3O6 · HCl
Quality: GMP, injectable grade
Development stage: validated
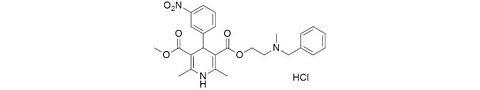
CAS Number: 7681-11-0
Molecular weight: 166,00 g/mol
Chemical formula: KI
Synonyms: –
Quality: GMP, injectable grade
Development stage: early, please contact us regarding timelines
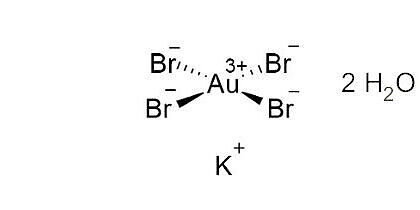
Potassium Tetrabromoaurate (III) dihydrate [13005-38-4]
CAS Number: 13005-38-4
Molecular weight: 591,71 g/mol
Chemical formula: KAuBr4 · 2 H2O
Quality: GMP, injectable grade
Development stage: early, please contact us regarding timelines
Selenious acid [7783-00-8]
CAS Number: 7783-00-8
Molecular weight: 128.97 g/mol
Chemical formula: H2SeO3
Synonyms: Selenous acid, Selenic (IV) acid
Quality: GMP, injectable grade
Development stage: validated
Selenium dioxide [7446-08-4]
CAS Number: 7446-08-4
Molecular weight: 110,96 g/mol
Chemical formula: SeO2
Synonyms: Selenium(IV) oxide, Selenous anhydride
Quality: GMP, injectable grade
Development stage: early, please contact us regarding timelines
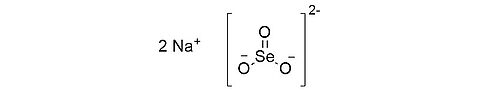
Sodium selenite (anhydrous) [10102-18-8]
CAS Number: 10102-18-8
Molecular weight: 172.94g/mol
Chemical formula: Na2SeO3
Quality: GMP, injectable grade
Development stage: validated
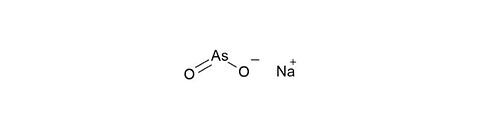
Sodium arsenite [7784-46-5]
CAS Number: 7784-46-5
Molecular weight: 129.92 g/mol
Chemical formula: NaAsO2
Synonyms: Sodium arsenate(III)
Quality: GMP, oral grade
Development stage: intermediate, please contact us regarding timelines
CAS Number: 7681-82-5
Molecular weight: 149,89 g/mol
Chemical formula: NaI
Synonyms: –
Quality: GMP, injectable grade
Development stage: early, please contact us regarding timelines
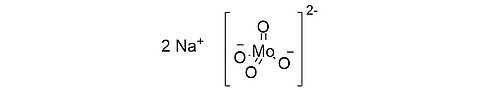
Sodium molybdate dihydrate [10102-40-6]
CAS Number: 10102-40-6
Molecular weight: 241.95g/mol
Chemical formula: Na2MoO4 ∙ 2 H2O
Synonyms: Molybdate sodium dihydrate
Quality: GMP, injectable grade
Development stage: validated
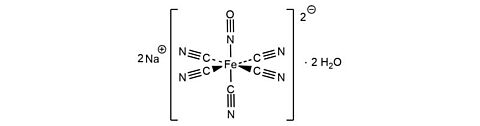
Sodium nitroprusside dihydrate [13755-38-9]
CAS Number: 13755-38-9
Molecular weight: 297.95 g/mol
Chemical formula: Na2[Fe(CN)5NO] · 2 H2O
Synonyms: Nitroprusside sodium,
Sodiumpentacyanidonitrosylferrat(II)-dihydrate,
Sodiumpentacyanonitrosylferrat(II)-dihydrate
Quality: GMP, injectable grade
Development stage: validated
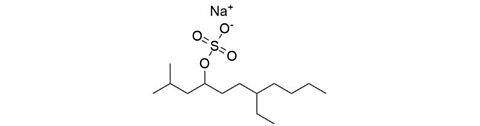
Tetradecylsulfate sodium (STS / STDS) [139-88-8]
CAS Number: 139-88-8
Molecular weight: 316.43 g/mol
Chemical formula: C14H29NaO4S
Synonyms: 7-Ethyl-2-methyl-4-hendecanol sulfate sodium salt,
Sodium 7-ethyl-2-methylundecyl-4-sulfate,
Sodium 2-methyl-7-ethylundecanol-4-sulfate
Quality: GMP, injectable grade
Development stage: validated
Zinc(II) chloride (anhydrous) [7646-85-7]
CAS Number: 7646-85-7
Molecular weight: 136.29 g/mol
Chemical formula: ZnCl2
Synonyms: Zinc chloride, Zinc dichloride
Quality: GMP, injectable grade
Development stage: validated
Zinc(II) sulfat monohydrate [7446-19-7]
CAS Number: 7446-19-7
Molecular weight:179.47 g/mol
Chemical formula: ZnSO4 x H2O
Synonyms: Zincsulfate
Quality: GMP, injectable grade
Development stage: early, please contact us regarding timelines
Zinc(II) sulfate heptahydrate [7446-20-0]
CAS Number: 7446-20-0
Molecular weight: 287.53 g/mol
Chemical formula: ZnSO4 x 7 H2O
Synonyms: Zincsulfate
Quality: GMP, injectable grade
Development stage: early, please contact us regarding timelines
Prices and availability are subject to requested volumes and requirements. All inquiries are subject to confirmation and any order subject to our terms andconditions of sale. Patented products available for research and development use, as permitted under CFR 35 section 271(e)(1) or directive 2001/82/EC anddirective 2001/83/EC (amendment 2004/27/EC) of the European Parliament. Patented products will not be shipped to countries with active patents thereof.
Your product is not on our list?
No problem! Our project chemists are pleased to offer you tailor-made custom development and manufacturing.

![[Translate to Englisch:] Strukturformel Selendioxid](/fileadmin/_processed_/b/8/csm_Selendioxid_1c16cb0ef0.jpg)